RESOURCES
Guidelines
- KDIGO (2012) – Clinical Practice Guideline for Acute Kidney Injury
- ICS (2009) – Standards and Recommendations for the Provision of Renal Replacement Therapy On Intensive Care Units In The United Kingdom
Review Articles
- Tolwani, Seminars in Dialysis (2009); Anticoagulation for Continuous Renal Replacement Therapy
- Schetz, Curr Op in Anasth (2001); Anticoagulation for Continuous Renal Replacement Therapy
- Macedo, AJKD (2016); Continuous Dialysis Therapies
- Gemmel, BJA Ed (2017); Renal Replacement Therapy in Critical Care
OBJECTIVES & QUESTIONS
Overview of Circuit Patency
Why does blood clot during RRT and what are the consequences? ·
- All modes of RRT (with the exception of PD) utilise an extracorporeal circuit
- Contact of blood with the foreign surface results in:
- Activation of both the intrinsic and the extrinsic pathway of coagulation
- Activation of platelets
- Exacerbated by pro-coagulopathic effects of AKI
- When the filter clots:
- Partially – the filter performance is reduced o
- Completely – the filter is lost and treatment us interrupted with loss of the filter, circuit and blood contained within
Pressures
Which pressures are measured in the CRRT circuit and what are the normal values?
What are the causes of abnormal access /return pressures?
- Low blood pump speed
- Line disconnection
- Pre-sensor line is clamped
- Catheter related:
- Kinked
- Against a vessel wall
- Occluded with clot
- Patient related – leading to poor flow:
- Hypovolaemia
- ↑ intrabdominal / intrathoracic pressure
- Hyperventilation
- Kinked
- Against a vessel wall
- Occluded with clot
- Dislodged (Emptying into a haematoma)
- Inadvertent arterial insertion
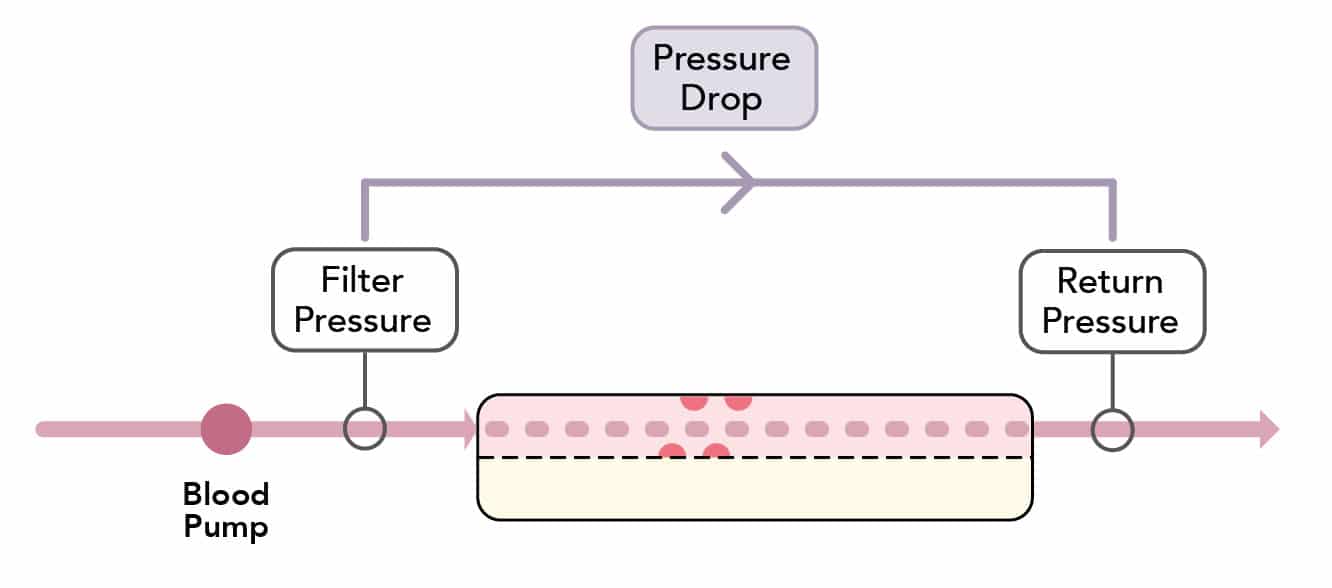
What is the pressure-drop?
- The ‘pressure drop’ is the pressure difference in the blood compartment across the membrane
- It reflects the ease of passage of blood through the filter:
Pressure~Drop~=~Filter~pressure~-~ Return~pressure
What causes increased pressure-drop?
- Increases in pressure drop occur when there is clotting of the membrane:
- Thrombosis on the intra-arterial side of the membrane preventing blood flow
- Usually occurs relatively early during continuous RRT
- Requires replacement of the filter
- A pressure-drop of >150-200 mmHg suggests a clotting filter
What are the causes of regular filter clotting?
- Access Issues:
- Poor vascular access
- Kinking of lines and tubes
- Circuit Issues:
- Bio-incompatible filter
- Post-dilution fluid replacement
- Reduced blood flows
- High filtration fraction
- De-aeration chamber insufficient
- Anticoagulation Issues:
- Poor anticoagulant choice/dose
- Coagulopathic patient
What is the transmembrane pressure (TMP)?
- The TMP is the pressure difference across the membrane between the blood and filtrate compartment
- It is estimated using a formula to give the pressure along the length of the filter:
Transmembrane~Pressure = \frac{Filter~pressure~+~Return~pressure}{2}~- ~Effluent~pressure
What are the causes of high transmembrane pressure?
- Increases in TMP occur when there is “clogging” of membrane pores:
- Formation of a “protein cake” occluding pores and preventing filtration through the membrane
- Results in increased pressure gradient needed to produce filtrate to a point that the membrane function becomes ineffective
- Usually occurs later in CRRT than “clotting”
- A TMP >300mmHg suggests that the filter set needs changing
- In the absence of evidence of a filter “clotting”, the filter should be washed back before changing to avoid excessive blood loss
Catheter Choice
What type of catheters should be used?
Catheters should be:
- Large diameter catheter (>11FG) – allowing blood flow in excess of 250 ml/min
- Polyurethane material
- Coaxial or lumen-in-lumen
- Uncuffed, non-tunnelled
Where should dialysis catheters be inserted?
The preference for insertion site should be:
- First choice: right jugular vein ·
- Second choice: femoral vein
- Third choice: left jugular vein
- Last choice: subclavian vein with a preference for the dominant side
What are the advantages and disadvantages of different insertion sites?
- Common technique that can allow good blood flows
- Right internal jugular lines are associated with the least recirculation and improved delivery of RRT compared with left
- Important structures nearby that are easily damaged
- Irritating to the awake patient
- Easy placement by both landmark and ultrasound techniques
- Associated with least complications that are often the least life-threatening
- Blood flows compromised by patient positioning
- Should sit in the inferior vena cava, this often necessitates the use of a line of 20-24cm in length
- Associated with higher rates of CRBSI in earlier studies though not confirmed by recent meta-analysis
- Most comfortable for patient
- High-complication rate due to landmark technique and risk of pneumothorax
- Blood flows often compromised by changes in ventilation
- High incidence of subclavian vein stenosis following large bore dialysis catheters
Where should the tip of the dialysis catheter lie?
- The position of the catheter tip improves flow:
- May be more important than catheter site
- KDIGO guidelines recommend the optimal site as:
- The junction of the superior vena cava and the right atrium
- Running parallel to the long axis to the SVC
- Positioning in the right atrium is advocated by some clinicians due to improved blood flow:
- Not advocated by KDIGO due to risk of atrial perforation and tamponade
- May be acceptable with softer tunnelled catheters
- Higher positioning in the brachiocephalic vein should be avoided as contact with the vessel wall occurs, occluding flow and increasing vessel thrombosis
Should a CXR be performed after insertion of a dialysis catheter?
- Whilst debated following CVC placement, chest x-ray is recommended after dialysis catheter insertion due to:
- Requirement of optimal positioning to achieve high blood flows
- Administration of anticoagulation
What can be done to troubleshoot a dialysis catheter with issues?
- Check position of catheter:
- Check chest x-ray
- Ensure in the SVC next to RA for upper sites / IVC for lower sites
- Change patient position
- Manipulate catheter:
- Flush lumens with saline
- Rotate the catheters
- Reverse catheter lumens
- Consider the use of fibrinolytic locks
- Prevent further issues:
- Careful clamp closing
- Use of anticoagulant locks (e.g. heparin)
Principles and Types of Anticoagulation
What are the advantages and disadvantages of anticoagulation in RRT?
- The goal of anticoagulation with RRT is to:
- Prevent clotting of the filter
- Prevent reduced membrane permeability
- This ensures adequate RRT can be achieved and prevents blood loss in the clotted filter
- Any benefits must be weighed against:
- Risk of bleeding
- Economic issues, such as workload and costs
What factors can increase the risk of circuits clotting?
- Poor vascular access
- Kinking of lines and tubes
- Reduced blood flows
- Convective rather than diffusive therapies
- Coagulopathic patient
- Poor anticoagulant choice/dose
- Bio-incompatible filter
What are the types of anticoagulation that can be used for RRT?
- Optimising CVP
- Pre-dilution replacement fluid
- High flowrates
- Reducing air-blood contact in the bubble trap
- Heparin
- Citrate
- Heparin
- LMWH
- Prostacyclin
- Thrombin Inhibitors:
- Argatroban
- Lepirudin
- Fondaparinux
- Heparinoids
- Warfarin
What are the advantages and disadvantages of each mode?
- Reduced bleeding risk
- ↓ cost
- ↑ risk of filter clotting:
- Shorter filter lifespan
- Reduced adequacy of RRT
- Not suitable for patients with HIT who are pro-thrombotic
- Easily titratable
- Easily monitored
- Can be reversed with protamine
- ↑ bleeding risk
- Risk of HIT
- ↓ cost
- ↑ familiarity
- ↑ bleeding risk
- Not titratable
- No reversal agent
- Reduced bleeding risk
- Shorter filter life
- Causes systemic hypotension
- Good regional anticoagulation with reversal by calcium
- Reduced bleeding risk
- Associated with metabolic complications (Hypernatraemia, hypocalcaemia, metabolic alkalosis)
- Special dialysate required
- Contraindicated in liver failure
- Labour intensive
Who should receive anticoagulation for RRT?
Anticoagulation should be used in all patients requiring RRT for AKI unless:
- There is an ↑ risk of bleeding
- They are already receiving systemic anticoagulation
Which mode of anticoagulation should be used during RRT?
- Regional citrate rather than heparin as first lineunless contraindicated
- If citrate contraindicated or as a second line alternative either unfractionated or low molecular weight heparin
- Regional citrate unless contraindicated
- Regional heparin should not be used
Which mode of anticoagulation should be used in Heparin Induced Thrombocytopenia (HIT)?
- All heparins should be stopped but anticoagulation should be continued during RRT as they are pro-thrombotic
- The direct thrombin inhibitor argobatran is recommended as first-line
- Other options include Factor Xa inhibitors such as danaparoid or fondaparinux
Monitoring Anticoagulation
How do you monitor anticoagulation during RRT?
- Visual Checks
- Access pressures
- Filtration fraction
- FUN/BUN ratio
- UFH: ACT post-filter / aPTT
- LMWH: anti-Xa
- Citrate: systemic and post-filter ionised calcium
- Prostacyclin: thromboelastography
- Lepuridin: aPTT
- Bivalirudin: apTT
- Bleeding
- Systemic anticoagulation
- HIT
- Metabolic problems
Mechanical Anticoagulation
Which patients can have RRT without pharmacological anticoagulation?
- Pharmacological anticoagulation is not required in patients:
- With impaired coagulation (e.g. liver failure or dilution coagulopathy)
- On systemic anticoagulation
- Impaired anticoagulation is suggested by any one of the following:
- INR >2-2.5
- APTT > 60 seconds
- Thrombocytopenia e.g. platelet count < 60 x 103/mm3
How can filter life be extended if no anticoagulation is used?
From KDIGO Guidance:
- Good vascular access allowing high blood flows to be reliably achieved
- Reducing blood viscosity through the filter
- Predilution fluids
- Treatments that involve diffusion as opposed to ultrafiltration
- Reducing blood-air contact in the bubble trap
- Ensuring prompt response to the filter alarms resulting in rapid correction of any suboptimal filter conditions which may have arisen
Other
- Prime the circuit with saline or heparin
- Give intermittent 0.9% saline flushes (50-200ml every 30-60mins)
Regional Heparin Anticoagulation
How does anticoagulation with UFH work?
- Anticoagulation with UFH uses an initial prime and subsequent infusion into the filter to prevent clotting
- Regular monitoring and titration of heparin rates ensures that systemic anticoagulation does not occur
- Remains the most commonly used anticoagulant in the UK due to its low costs, familiarity and ease of administration
What are the advantages of UFH?
- Effective
- Widely Available
- Easily titratable
- Easily monitored
- Can be reversed with protamine
- Inexpensive
- Short Half-life
What are the disadvantages of UFH?
- ↑ bleeding risk
- Unpredictable kinetics
- aPTT not reliable predictor of bleeding risk
- Heparin resistance can occur
- Risk of HIT
How should you manage a patient requiring UFH?
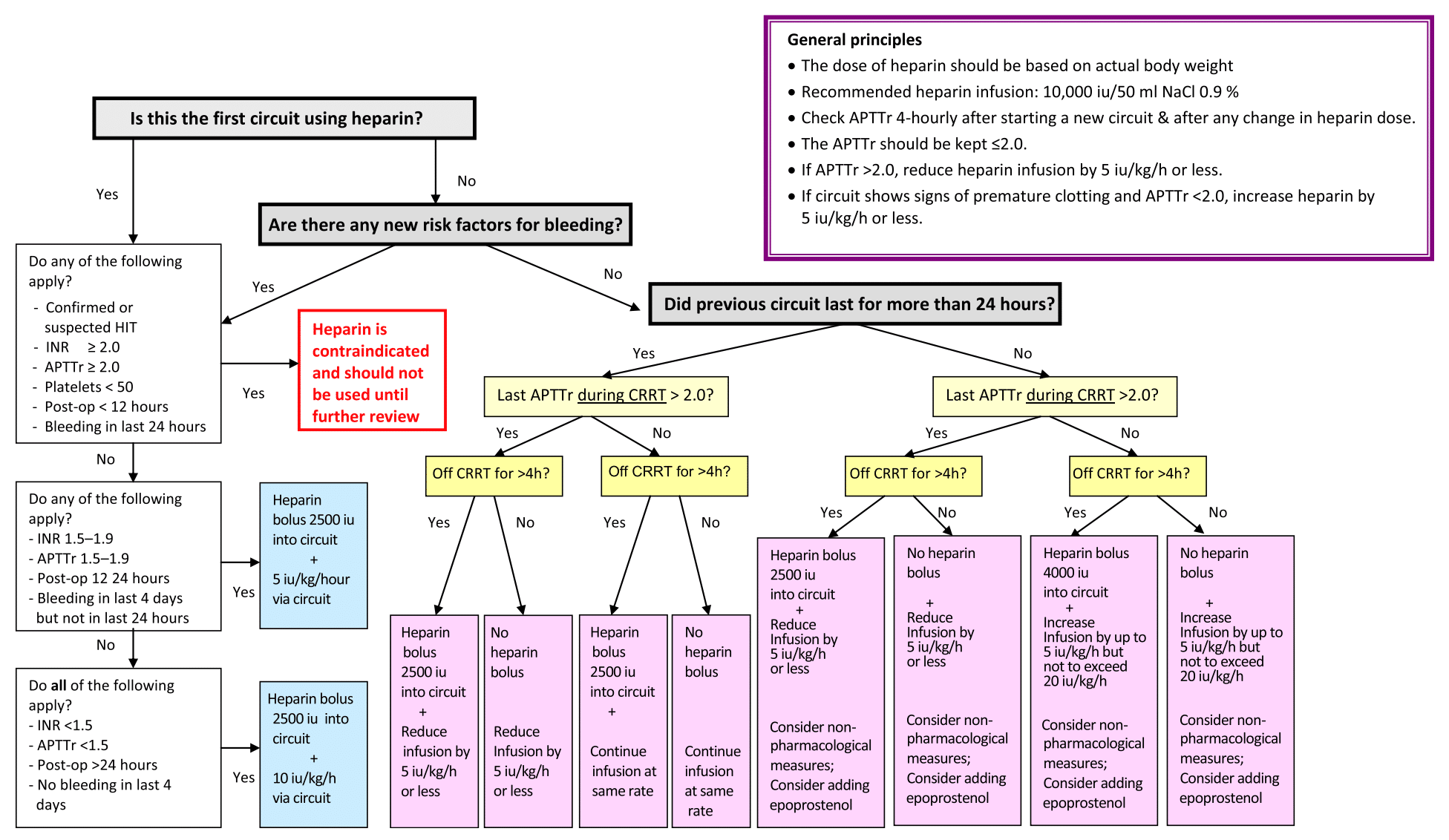
- Each unit will have its own individual policy on how to manage regional heparin anticoagulation via a filter
- A suggested algorithm has been published:
Regional Citrate Anticoagulation
How does citrate anticoagulation work?
- Calcium (factor IV) is an essential substrate for all 3 clotting pathways
- Citrate is added to blood prior to the filter in the form of trisodium citrate:
- Chelates calcium forming a complex
- Prevents the involvement of calcium in the clotting cascade
- Regional anticoagulation of the filter only is ensured through several mechanisms:
- Citrate-calcium complexes are small molecules and freely pass through the filter (via diffusion or convection)
- Residual citrate in the blood is delivered to the patient and metabolised by the liver to bicarbonate
- Calcium ions are infused post-filter to replace that lost in effluent, normalising levels and clotting function
What are the advantages of citrate anticoagulation?
- Superior anticoagulation to UFH
- Prolonged filter lifespan
- Avoidance of any systemic anticoagulation reducing bleeding complications
What are the disadvantages of citrate?
- Cumbersome protocol; greatly ↑ workload
- Requires frequent monitoring
- Can be associated with significant metabolic derangement or toxicity
- Special calcium-free hyponatremic and bufferless dialysate is required
- Contraindicated in liver failure (citrate is primarily hepatically metabolised)
- Systemic anticoagulation still required in patients with HIT
What are the complications of citrate anticoagulation?
- Hypocalcaemia – due to chelation
- Hypomagnesaemia – Mg2+ is also chelated
- Hypernatraemia – high sodium load in hyperosmolar trisodium citrate
- Metabolic alkalosis – citrate is metabolised to bicarbonate
- Metabolic acidosis (RAGMA) – caused by the citrate accumulation if there is impaired metabolism (e.g. liver failure)