OBJECTIVES & QUESTIONS
Indications
What are the indications for renal replacement therapy?
Renal Indications
Emergency Indications
- Metabolic
- ↑ K+ (>6.5 mmol/L)
- ↑ urea (>40 mmol/L)
- Uraemia with symptoms:
- Pericarditis
- Encephalopathy
- Refractory pulmonary oedema
- Metabolic Acidaemia due to renal failure (pH<7.15)
- Oligo-anuria(<200ml/24 hours)
Non-Emergency Indications
- Acheivement of homeostasis in AKI unresponsive to conservative management
- Acid-base balance
- Solutes
- Fluid
Non-Renal Indications
- Removal of dialysable toxins
- Removal of contrast agent (Less relevant with newer contrast agents)
- Clearance of cytokines to decrease severity of sepsis (controversial)
- Control of body temperature (an extracorporeal circuit can help control hypo or hyperthermia which is resistant to other methods of control)
- Control of otherwise uncontrollable electrolytes:
- ↑ Ca+ refractory to pamidronate
- Sodium abnormalities resistant to treatment
What are the characteristics of drugs that can be cleared by RRT?
- Low volume of distribution
- Low molecular weight
- Low protein binding
- Low water solubility
What are examples of drugs that can be cleared by RRT?
Memory Tip
Can be remembered using the mnemonic:
I STUMBLE
- Isopropyl
- Salicylates
- Theophylline
- Urea
- Methanol
- Barbiturates and anti-convulsants:
- Phenobarbital
- Carbamazepine
- Phenytoin
- Lithium
- Ethylene Glycol & other alcohols
Others include:
- Metformin
- Methotrexate
- Paraquat
- Mushroom toxins
- Antibiotics:
- Most penicillins
- Cephalosporins
- Carbapenems, aminoglycosides, metronidazole
- Some beta-blockers: sotalol, atenolol
Timing
When should RRT be initiated in AKI?
- To manage emergency indications
- To achieve fluid, electrolyte, acid-base homeostasis in AKI unresponsive to conservative treatment (Theoretical benefits must outweigh potential drawbacks)
In the absence of emergency indications, what are the advantages and disadvantages with early and late timing of RRT initiation?
Early Initiation
- May confer benefit, in particular in circumstances in which there is a perception that recovery from AKI is not imminent
- Can theoretically facilitate more rapid correction of electrolyte and acid-base derangements and control of uraemia and mitigate fluid accumulation
- Would prevent the occurrence of overt complications of AKI
- May limit worsening of non-renal organ dysfunction (potential kidney-organ interactions)
- Will result in initiation of RRT in a significant portion of patients who may have regained renal function with conservative management
Late Initiation
- Has not been shown to lead to worse outcomes than early initiation
- Prevents complications of RRT in subset of patients that did not require it:
- Insertion of dialysis catheters
- Exposure to extracorporeal circuits
- Clearance of nutrients and medication
- Iatrogenic haemodynamic instability
- Reduces healthcare costs by limiting bedside workload and resource utilisation
What is early or late initiation?
- There is no consensus definition for early or late timings of RRT
- A wide variety of arbitrary definitions exist based upon:
- Physiologic parameters (e.g., urine output)
- Biochemical parameters (e.g., serum creatinine, urea)
- Time relative to the development of AKI (also variably defined)
- Time relative ICU admission
- The heterogeneity in definitions creates difficulty for interpreting studies and determining optimal clinical practice
What is the evidence in regard to early or late initiation of RRT?
Intervention
Population
Conclusion
- Early vs. Late initiation of RRT
- Early: Within 8 hours of meeting KDIGO stage 2 AKI
- Late: Within 12 hours of meeting KDIGO stage 3 AKI or emergency indication
- 231 patients with AKI and either severe sepsis or requiring catecholamine infusion
- Early group showed significantly lower mortality at 90 days (39.3% vs. 54.7%, p=0.03)
- Early vs. Late initiation of RRT
- Early: Immediately upon meeting KDIGO stage 3 AKI criteria
- Late: If oliguric 72 hours after meeting KDIGO stage 3 AKI criteria or emergency indications
- 620 patients with AKI requiring mechanical ventilation or catecholamine infusion
- No difference in mortality between early and late group (48.5% vs 49.7%, p=0.79)
- In delayed group 49% did not require RRT
- Early vs. Late initiation of RRT
- Early: within 12-hours of meeting failure by RIFLE criteria
- Late: after 48-hours of meeting failure by RIFLE criteria if no renal recovery or emergency indications
- 488 patients with severe AKI and septic shock
- No difference in mortality between early and late group (58% vs 54%, p=0.38)
- In delayed group:
- 38% did not require RRT
- 17% met indications for emergency RRT
When should RRT be initiated in the absence of emergency indications?
- In the absence of emergency indications:
- Current evidence does not firmly confirm advantages of either an early or late initiation of RRT
- Most evidence supports the use of a “wait and see” attitude without leading to worse outcomes
- Patient should be considered as a whole (Table adapted from Macedo et al):
Severity of Illness & Trajectory
- AKI severity and trend
- Severity of electrolyte and acid base disorder
- Fluid balance and symptoms of overload
- Presence of other organ dysfunction impacted by AKI / fluid overload
Necessity of RRT
- Likelihood of early recovery of kidney function without RRT
- Underlying comorbidities impacted by AKI / fluid overload
- Associated acute organ dysfunction
Risks of RRT
- Vascular access
- Haemodynamic instability
- Infection
- Clearance of trace elements / vitamins / drugs
- Immobilisation
Other Factors
- Patient and family wishes
- Overall goals of care
- Availability of machines and nursing staff
- Healthcare costs
What do current guidelines state on the timing of initiation?
Recommendation
KDIGO
- Initiate RRT emergently when life-threatening changes in fluid, electrolyte, and acid-base balance exist
- Consider the broader clinical context, the presence of conditions that can be modified with RRT, and trends of laboratory tests rather than single BUN and creatinine thresholds alone when making the decision to start RRT
NICE
- Refer adults, children, and young people immediately for RRT if any of the following are not responding to medical management:
- ↑ K+
- Metabolic acidosis
- Complications of uraemia (i.e., pericarditis, encephalopathy)
- Fluid overload
- Pulmonary oedema
- Base the decision to start RRT on the condition of the adult, child, or young person as a whole and not on an isolated urea, creatinine, or potassium value.
Cessation
When should RRT be stopped?
- Guidance suggests RRT can be stopped when it is no longer required due to:
- Intrinsic recovery of kidney function
- Treatment no longer consistent with goals of care (withdrawal)
- However, determining when intrinsic function is adequate is difficult and no clear consensus exists
Which features suggest adequate recovery of renal function?
- The patient should be on a globally improving trend
- Several markers have been suggested for demonstrating renal recovery
- Urine volume whilst on RRT:
- A urine output >400ml in 24 hours gives an 80% chance of success at liberating from RRT
- Diuretics should not be used to enhance urine output
- Creatinine clearance whilst on RRT:
- Solute clearance stabilises after 48 hours on RRT allowing reliable measurement of creatinine clearance
- CrCl of >20ml/min should to be predictive of successful liberation
- Rarely used as a marker in clinical practice
- Urine volume whilst on RRT:
- An alternative strategy is to take a ‘filter holiday’ and monitor trends in function
Components
What are the basic components of CRRT that need prescribing in ICU?
Choice
Blood Flow Rate
- Blood flow rates are typically slower than in intermittent dialysis (150-200mL/min)
- Generally, the faster the flow, the more efficient the dialysis.
Dialysate or Replacement Fluid Composition
- The specific fluid is based on the metabolic parameters of the patient, including the patient’s acid-base status and serum potassium concentration
- Typical flow rates range from 500mL/min to 800mL/min
Replacement Fluid Pre/Post Dilution Ratio
- The proportion of replacement fluid delivered before or after the filter
- Typically started with 30% pre- and 70% post-dilution
Effluent Rate (Dose)
- Dosing is weight based and is typically prescribed at a dose ranging from 20 mL/kg/hr to 35 mL/kg/hr
Fluid Removal Goal
- This is the amount of fluid to be removed from the patient over the course of the session
- Determined by clinical assessment of the patient’s volume status.
Anticoagulation
- Clotting within the dialysis circuit can result in significant blood loss
- Heparin is typically used unless the patient has a contraindication
- An alternative to heparin anticoagulation often used with CRRT is regional citrate anticoagulation, in which citrate is administered to chelate calcium, a critical cofactor in the clotting cascade
Dose & Efficacy
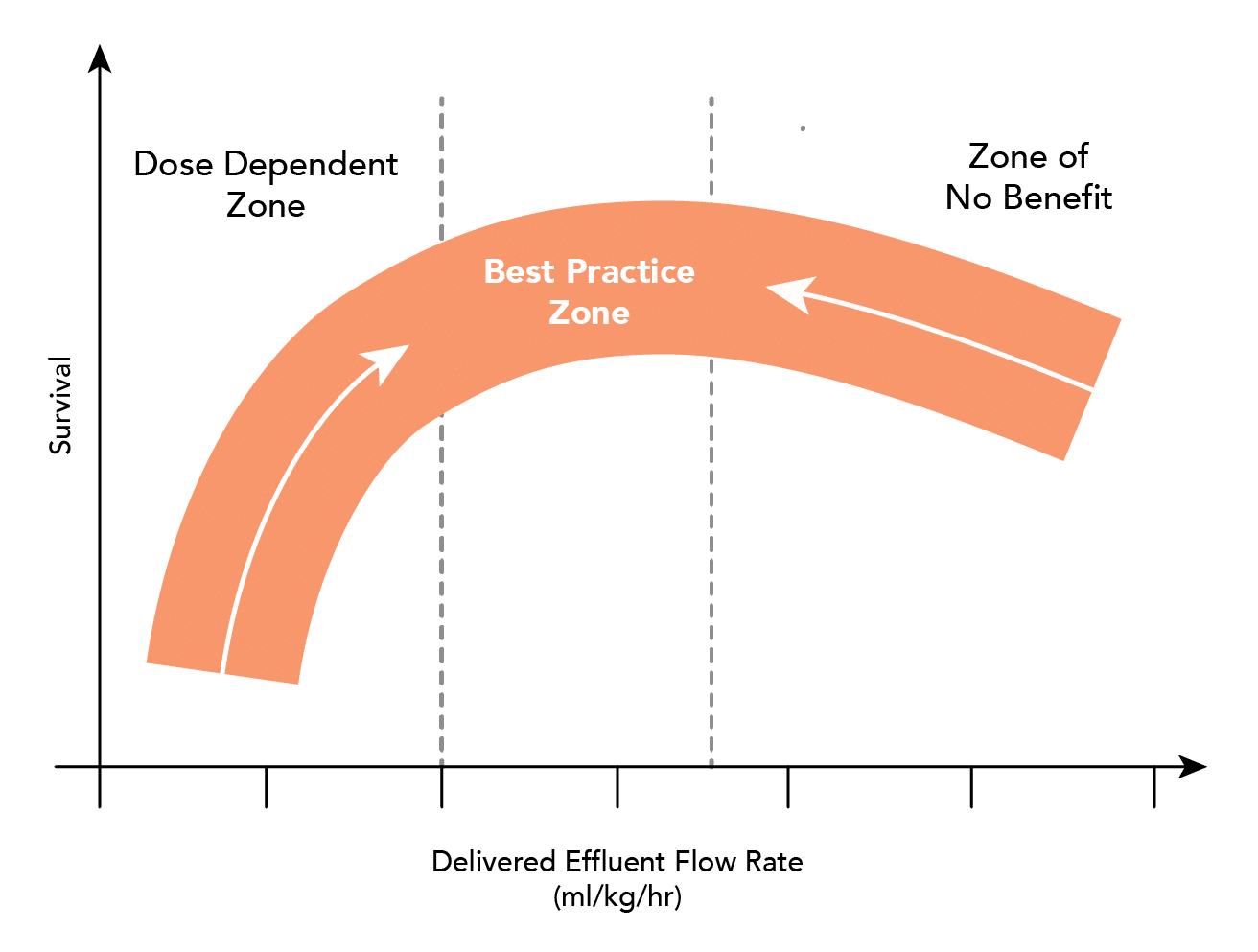
What is the optimal dose of CRRT in AKI?
- Adequate clearance, and how this influences outcome is not clear:
-
- Current research suggests that a rate of 20-25ml/kg is adequate
- Higher rates of clearance (35-40ml/kg) confer no benefit
- Higher rates may need to be prescribed to ensure minimum desired clearance is met
- High volume hemofiltration may be of benefit in:
- Rhabdomyolysis
- Clearance of ammonia and lactate
- Pancreatitis (experimental)
- Clearance of cytokines (experimental)
What evidence exists to determine the optimum ‘dose’ of CRRT?
Intervention
Population
Conclusion
- Early vs. Late initiation of RRT
- Early: Within 8 hours of meeting KDIGO stage 2 AKI
- Late: Within 12 hours of meeting KDIGO stage 3 AKI or emergency indication
- 231 patients with AKI and either severe sepsis or requiring catecholamine infusion
- Early group showed significantly lower mortality at 90 days (39.3% vs. 54.7%, p=0.03)
- Early vs. Late initiation of RRT
- Early: Immediately upon meeting KDIGO stage 3 AKI criteria
- Late: If oliguric 72 hours after meeting KDIGO stage 3 AKI criteria or emergency indications
- 620 patients with AKI requiring mechanical ventilation or catecholamine infusion
- No difference in mortality between early and late group (48.5% vs 49.7%, p=0.79)
- In delayed group 49% did not require RRT
- Early vs. Late initiation of RRT
- Early: within 12-hours of meeting failure by RIFLE criteria
- Late: after 48-hours of meeting failure by RIFLE criteria if no renal recovery or emergency indications
- 488 patients with severe AKI and septic shock
- No difference in mortality between early and late group (58% vs 54%, p=0.38)
- In delayed group:
- 38% did not require RRT
- 17% met indications for emergency RRT
What is the difference between prescribed and delivered dose?
- There may be a significant difference between the prescribed ‘dose’ and that which is actually delivered – estimated using clearance equations to be 73% in practice
- Due to a number of factors:
- Treatment downtime due to filter clotting
- Technical problems such as boor blood flow and recirculation
- Reduced filter efficacy over time
- Effects of pre-dilution
What dose should be prescribed?
- It is recommended that a dose of at least 35 ml/kg/h (post-dilution) is prescribed for CRRT
- This ensures that an adequate dose of CRRT is delivered despite downtimes and other limiting factors
Why has a higher doses not shown to improve outcomes?
- Increasing the dose may cause loss of essential molecules and thus affect outcome:
- Antibiotics – many significantly cleared by RRT
- Amino acids and proteins
- Micronutrients (vitamins, selenium, folic acid)
Complications & Troubleshooting
What are the complications of RRT?
Access Related
- Failure to obtain access
- Damage to vessels and surrounding structures
- Risk of venous stenosis
- Haemorrhage
- Infection
- Air Embolism
- Pain
Anticoagulation Related
- Bleeding
- Heparin induced thrombocytopenia
- Hypocalcaemia with citrate
Filtration Related
- Hypotension and haemodynamic instability:
- Common on initiation of renal replacement as the circuit often takes 200-300 ml of blood
- Usually responds to a fluid bolus
- Removal of solutes:
- Electrolyte imbalance
- Trace elements and amino acids
- Therapeutic drugs
- Metabolic abnormalities
- Reaction to filter membrane and anaphylaxis
- Rising lactate:
- Inability to metabolise lactate in the filter fluid to bicarbonate
- Switch to filter fluid with bicarbonate buffer
Prognosis Following RRT
What is the prognosis of patients requiring RRT in ICU?
- Mortality remains high in critically ill patients
- Mortality around – 45-60% in studies
- RRT withheld in around 20% of patients
- Of those that survive to ICU discharge:
- If no chronic renal impairments prior to admission:
- Long-term RRT required in 2-11%
- CKD in 44%
- If chronic renal impairment prior to admission:
- Long-term RRT required in up to 55%
- If no chronic renal impairments prior to admission:
Pharmacokinetics & Drug Dosing
Which factors affect pharmacokinetics during RRT?
Drug Factors
- Protein binding:
- Highly protein bound drugs (e.g. warfarin, diazepam, propranolol and phenytoin) are only cleared by RRT in small amounts
- Molecular weight:
- Larger molecules cleared less effectively by diffusive therapies
Therapy Factors
- Timing of RRT:
- Drugs given between sessions will not be cleared until the subsequent session
- Dose of RRT:
- Reduced flow rates / shorter sessions will decrease clearance of drugs
- Membrane permeability
Patient Factors
- Residual GFR and urine production
- Hypoalbuminaemia
How should drugs be dosed during RRT?
- Dosing during RRT can be challenging given the numerous factors needed to be accounted for:
- Should not simply be dosed for reduced GFR
- Nomograms developed for use in stable patients on IHD can result in significant under-dosing
- Drug levels should be measured to aid dosing whenever possible
- In the absence of drug levels:
- ‘Bedside’ dosing guidelines should be used (e.g. ‘Renal Drug Handbook’)
- Expert pharmacist help should be sought when possible