RESOURCES
Guidelines
- KDIGO (2012) – Clinical Practice Guideline for Acute Kidney Injury
- ICS (2009) – Standards and Recommendations for the Provision of Renal Replacement Therapy On Intensive Care Units In The United Kingdom
Review Articles
- Macedo, AJKD (2016); Continuous Dialysis Therapies
- Neri, Crit Care (2016); Nomenclature for renal replacement therapy in acute kidney injury: basic principles
- Gemmel, BJA Ed (2017); Renal replacement therapy in critical care
- Honore, J Transl Intern M (2018); What a clinical should know about a renal replacement membrane?
OBJECTIVES & QUESTIONS
Introduction
What is renal replacement therapy?
- Renal replacement therapy (RRT) describes techniques used to purify the blood and achieve the solute and fluid homeostasis usually produced by the kidney
- Continuous renal replacement therapy (CRRT) describes treatments which are applied for prolonged periods at a time (usually >24 hours)
How can modes of renal replacement be classified?
Modality of Fluid and Solute Removal
- Hemofiltration
- Haemodialysis
- Hemodiafiltration
Continuous vs. Intermittent Modality
- Intermittent:
- Intermittent haemodialysis (IHD)
- Slow low-efficiency dialysis (SLED)
- Extended daily dialysis (EDD)
- Continuous:
- Continuous venovenous hemofiltration (CVVH)
- Continuous venovenous haemodialysis (CVVHD)
- Continuous venovenous hemodiafiltration (CVVHDF)
- Slow continuous ultrafiltration
- Peritoneal dialysis (PD)
Source of Pressure Gradient
- Arteriovenous
- Venovenous - requires an external pump
Mechanisms
What are the mechanisms used for fluid and solute removal in renal replacement, and how are they defined?
Definition
Diffusion
The transport of solute across a semi-permeable membrane, down a concentration gradient
Ultrafiltration
The passage of fluid across a semi-permeable membrane due to a hydrostatic pressure
Convection
The transport of a solute across a semi-permeable membrane along with solvent (by "solvent drag")
What are the characteristics of molecules cleared by RRT?
- Small volume of distribution (<1L/kg)
- Low degree of protein-binding
- High water solubility
- Low molecular weight:
- Only smaller molecules (up to 500 Da) are cleared by hemodialysis
- Up to a maximum 40 kDa can be cleared by hemofiltration
- Low endogenous clearance (<4 ml/min/kg)
- Extraction ratio exceeding endogenous elimination
What factors influence the clearance of a substance from plasma?
- Membrane properties:
- Surface area of membrane
- Hydraulic permeability
- Pore size
- Charge
- Pressure gradients: hydrostatic, colloid, osmotic
- Solute properties: size, charge, concentration
Modality Principles
What are the modalities of RRT?
Mechanisms
Haemodialysis
- Clearance of solutes via diffusion down a concentration gradient
- A counter-current flow of a solution containing various electrolytes on the opposite side of membrane to blood allows diffusion to occur
- No fluid is added to the filtrate after diffusion
- Rate of solute clearance determined by:
- Concentration gradient between plasma and dialysate
- Particle size, ionic charge and protein binding
- Membrane pores, thickness and surface area
Haemofiltration
- Clearance of solutes via convection driven by hydrostatic pressure
- Filtrate removal is balanced by the addition of a solution to maintain volume
- Does not significantly change the concentration of serum electrolytes and waste products unless a replacement fluid is infused into the blood, effectively diluting out those solutes the physician wishes to remove
- Rate of filtrate and solute removal determined by:
- Blood flow
- Transmembrane pressure gradient
- Membrane coefficient (pore size/permeability)
Haemodiafiltration (Combined)
- Clearance of solute via convection and diffusion
- Countercurrent dialysate is used in addition to hydrostatic pressure
- Fluid replacement is required to maintain plasma volumes
How does the modality effect solute clearance?
Size
Examples
Removal
Small Molecules
<500 Da
- Urea
- Creatinine
- Potassium
- Oxalate
- Uric acid
- Better cleared by diffusion (Haemodialysis)
- Rate of clearance increased by:
- Increasing speed of dialysis fluid flow
Middle Molecules
500 - 60,000 Da
Albumin is 66 kDa (Not a middle molecule!)
Albumin is 66 kDa (Not a middle molecule!)
- Interleukins
- Cytokines
- B2 -microglobulin
- ANP
- TNF
- Light chains
- Better cleared by convection (Hemofiltration)
- Dialysis clearance increased by:
- Increased dialysis time
- Increased membrane pore size
- Increased membrane surface area
Protein-Bound Molecules
Variable
- Homocysteine
- Hippuric acid
- Phenol
- Difficult to remove via RRT
- Clearance improved by:
- Increased time and flow
- Absorbent technology
- Albumin dialysis
Modality Choice
Which factors influence the choice of modality that should be used?
Solutes to be Removed from the Plasma
- Hemofiltration is better at removing middle molecules
- Hemodialysis better at removing small molecules
Patient`s Cardiovascular and Neurological Status
- CRRT causes less rapid fluid shifts and is the preferred option if there is any degree of cardiovascular instability
- CRRT may be associated with better cerebral perfusion in patients with an acute brain injury or fulminant hepatic failure
Availability of Resources
- CRRT is more labour intensive and more expensive than IHD
- Availability of equipment may dictate the form of RRT.
Clinician`s Experience
- It is wise to use a form of RRT that is familiar to all the staff involved
Other Specific Clinical Considerations
- Convective modes of RRT may be beneficial if the patient has septic shock
- CRRT can aid feeding regimes by improving fluid management
What are the advantages and disadvantages of different RRT modalities?
Advantages
Disadvantages
Requirement
Intermittent Haemodialysis
- Efficient and intensive technique
- Allows for down-time for interventions
- Can be performed overnight
- Staff required for a shorter time
- Lower costs
- May cause haemodynamic instability with rapid fluid removal
- Relative need for anticoagulation
- Potential for disequilibrium
- Need for expensive machinery
- Need for personnel
- Venous access
- Anticoagulation
- Skilled staff
- Expensive equipment
Continuous RRT
- Provides better haemodynamic stability
- Efficient solute removal and electrolyte balance with continuous removal
- Round-the-clock maintenance of volume status
- Nutrition and medication given while volume status maintained
- User-friendly machines
- Patient immobilisation
- Need for prolonged anticoagulation
- Nursing staff intensive
- Expensive machinery
- Risk of hypothermia
- Round the clock skilled nursing staff
- Venous access
- Anticoagulation
- Complex equipment
Peritoneal Dialysis
- Better haemodynamic stability than haemodialysis
- No need for anticoagulation
- Slow correction of volume and electrolyte disorders
- Many nurses unfamiliar with methods
- Risks of leaks and peritoneal infection
- Peritoneal catheter
- Sterile peritoneal solutions
- Trained staff
Which modality is better for critically unwell patients?
- There is no evidence that the use of either continuous or intermittent therapies have a survival benefit in critical illness
- Continuous modalities have been Several benefits have been proposed from the use of continuous modalities:
- Improved haemodynamic stability and lower rates of therapy-induced hypotension – due to slower and more predictable rates of fluid removal and solute flux
- Increased clearance with continuous modes to aid with the resolution of uraemia and electrolyte imbalance
- Better tolerated in patients with raised intracranial pressure or hepatic encephalopathy due to less rapid shift in solute concentration and preservation of cerebral perfusion
- Better clearance of inflammatory mediators
- Given this international guidance favours the use of continuous therapies in critical illness
- KDIGO guidance recommends continuous therapies in ‘haemodynamically unstable patients’ or those with ‘raised ICP, brain injury or other forms of brain oedema.’
- Surviving sepsis guidance recommends continuous therapies in ‘haemodynamically unstable septic patients
Specialist Modalities
What is SCUF?
- Provision ultrafiltration to remove excess fluid – mainly at the end of a dialysis session
- It is useful solely for fluid removal and does not alter the patient’s biochemistry
What are hybrid therapies?
- Hybrid therapies refer to a collective of recently developed therapies which includes:
- SLED / EDD – Slow Low-Efficiency Daily Dialysis / Extended Daily Dialysis
- SCD – Sustained Continuous Dialysis
- Most therapies delivered via conventional dialysis machines over a more extended period than usual IHD
- Hybrid therapies benefit from being intermittent, but also are associated with
less haemodynamic and osmotic disturbance than intermittent HD, and good solute control
What is SLED?
- SLED is an intermittent therapy typically delivered over 6-12 hours
- It typically uses lower blood and dialysate flow rates using conventional dialysis machines
- The advantaged of SLED are:
- Improved haemodynamic stability
- High solute clearance
- Removes the need for expensive CRRT machines and customized solutions
- Allows for diagnostic and therapeutic procedures
Dose & Efficacy
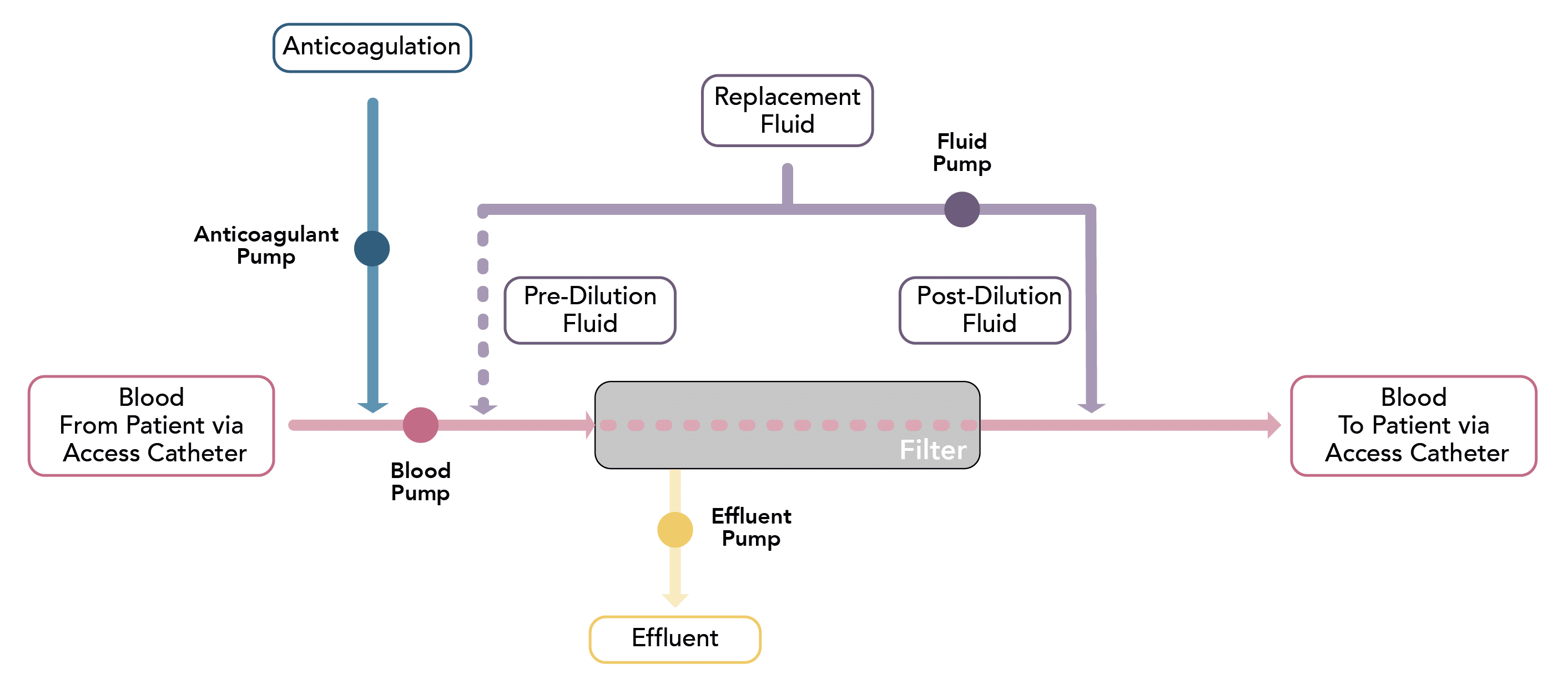
Which variables, that influence its function, are controlled through the renal replacement circuit?
Blood Flow Rate
Blood Flow Rate
Qb
- The rate of blood flow from the patient towards the filter
- Blood flow rates are typically slower than in intermittent dialysis, ranging from 150-200mL/min
Ultrafiltrate Rate
Ultrafiltrate Rate
Quf
- The rate at which ultrafiltrate is produced by hydrostatic pressure across the membrane during convective therapies
- It is influenced by the blood flow rate and the filtration fraction
Dialysate Flow Rate
Dialysate Flow Rate
Qd
- The flow rate of dialysis fluid in dialytic therapies
- Standard flow rates range from 8-50 mL/min
Effluent Rate
(Dose)
(Dose)
Effluent Rate
(Dose)
(Dose)
Qef
- The effluent flow rate is the equivalent to ultrafiltration rate Quf in continuous haemofiltration, Qd in continuous haemodialysis, and both Quf & Qd in continuous hemodiafiltration
- It is analogous to the ‘dose’ in continuous therapies
- Dosing is weight-based and is typically prescribed at a dose ranging from 20 mL/kg/hr to 35 mL/kg/hr
Substitution Fluid Rate
Substitution Fluid Rate
Qs
- Techniques in which produce a volume of ultrafiltrate require replacement of this fluid with a substitute fluid to prevent significant loss of volume.
- The rate of substitution fluid describes the rate at which this replacement fluid is added to the plasma
Net Ultrafiltrate Rate
Net Ultrafiltrate Rate
Qnet
- Represents the overall rate of fluid removal
- It is the difference between the total effluent removed (Qef) minus the volume of replacement therapy
- The fluid removal rate can be tailored by the machine to meet the total removal goal of the patient
What determines the clearance in CRRT?
- Clearance describes the volume of blood that is cleared of a substance per unit of time
- The determinant of clearance in CRRT depends upon the mechanisms that are being utilised
- In continuous haemofiltration:
- A volume of ultrafiltrate is produced following the passage of the pressurised plasma over the membrane
- Clearance is of a solute determined by the:
- Rate at which ultrafiltrate is produced (Quf)
- Sieving coefficient of the filter membranes (determined by the ratio of solute concentrations in the ultrafiltrate)
- Most small molecules have a sieving coefficient of 1, meaning they pass freely through the membrane (though this is less true of middle molecules)
- Therefore, the clearance is analogous to the ultrafiltrate production rate (Quf)
- In continuous haemodialysis:
- The concentration gradient across the membrane determines clearance
- The gradient is affected by the dialysate flow rate (Qd) and the blood flow rate (Qb)
- Qd is much slower than Qb, and the dialysate becomes fully saturated.
- Therefore, the dialysate rate (Qd) becomes the rate-limiting factor for solute removal and is analogous to clearance
- Overall consequently, clearance is equivalent to the combination of the ultrafiltration rate (Quf) and dialysate (Qd), which is termed the effluent rate (Qef)
- This is often discussed in terms of the ‘dose’ of a continuous replacement therapy which is given
- In continuous haemofiltration:
How is the effluent rate determined?
- In ultrafiltration, the effluent rate is determined by the flow of plasma into the membrane and the fraction of this which is filtered out as effluent (known as the filtration fraction)
- To maintain a constant effluent rate:
- At low blood flows a large filtration fraction is required
- At high flows, a small filtration fraction can be used
- The renal replacement circuits have inbuilt algorithms to deliver a desired effluent flow rate based upon the blood flow from the patient
What effect do changes in the blood flow rate have?
- Normal filter blood flows range between 50-200mL/min
- Choosing the appropriate flow is a trade-off between the advantages and disadvantages of high and low flows:
Advantages
Disadvantages
High Blood Flows
- A lower filtration fraction required thus reducing the risk of filter clotting
- Easier to match ultrafiltration rates and therefore fluid removal targets
- Increased risk of haemodynamic instability
- Increased risk of hypothermia or haemolysis
- Requires well-functioning access to prevent high suction pressures developing
- Increased risk of filter clotting due to high filtration fractions
- More challenging to meet fluid removal targets
Low Blood Flows
- Reduced haemodynamic instability
- Reduced risk of filter related complications
- Lower suction pressures needed to achieve flow rates
- Increased risk of filter clotting due to high filtration fractions
- More challenging to meet fluid removal targets
What effect do changes in the filtration fraction have?
- The filtration fraction is a measure of haemoconcentration in the filter
- It is defined by the ratio between volume removed by convection per volume of plasma flow
- If the ratio is too high, the filter tends to clot – more concentrated blood is ‘stickier’ and more likely to coagulate
- Generally aiming to keep the filtration ratio below 25-30% will prevent clotting in the filter.
Membranes
What are the properties of the membrane that impact the function?
Biocompatibility
- The rate of blood flow from the patient towards the filter
- Blood flow rates are typically slower than in intermittent dialysis, ranging from 150-200mL/min
Flux
- The rate at which ultrafiltrate is produced by hydrostatic pressure across the membrane during convective therapies
- It is influenced by the blood flow rate and the filtration fraction
Adsorption
- The flow rate of dialysis fluid in dialytic therapies
- Standard flow rates range from 8-50 mL/min
Thickness
- The effluent flow rate is the equivalent to ultrafiltration rate Quf in continuous haemofiltration, Qd in continuous haemodialysis, and both Quf & Qd in continuous hemodiafiltration
- It is analogous to the ‘dose’ in continuous therapies
- Dosing is weight-based and is typically prescribed at a dose ranging from 20 mL/kg/hr to 35 mL/kg/hr
Surface area
- Techniques in which produce a volume of ultrafiltrate require replacement of this fluid with a substitute fluid to prevent significant loss of volume.
- The rate of substitution fluid describes the rate at which this replacement fluid is added to the plasma
Material
- Represents the overall rate of fluid removal
- It is the difference between the total effluent removed (Qef) minus the volume of replacement therapy
- The fluid removal rate can be tailored by the machine to meet the total removal goal of the patient
Which membranes are used in CRRT?
- There is no conclusive evidence that any specific membranes improve the outcome
- In practice, most filters used for CRRT are:
- Synthetic, high-flux membranes
- Surface area of 0.6–1.2m2
- Pore size allowing the passage of molecules up to 50,000 Daltons