RESOURCES
Review Articles
- Meekha, Mayo Clin Proc (2011); General Principles of Antimicrobial Therapy
- Denny, Clin Micro & Inf (2019); When not to start antibiotics: avoiding antibiotic overuse in the intensive care unit
- Masterton, Crit Care Clin (2011); Antibiotic De-Escalation
- Enzler, Mayo Clin Proc (2011); Antimicrobial Prophylaxis in Adults
OBJECTIVES & QUESTIONS
Introduction & Definition
What is an antibiotic?
- An antibiotic is chemical substance that can inhibit the growth of or destroy bacteria without damaging the host cell at therapeutic levels
- Traditionally the term referred to substances produced by a living organism but today it is used more broadly to include any compound, whether of natural or synthetic origin
By which ways can antibiotics be classified?
There are a number of ways by which antibiotics may be classified:
- By effect against bacterial cells (e.g. bacteriocidal or bacteriostatic
- By mechanism of action
- By structure
- By spectrum of activity (e.g. narrow or broad)
What effect can antibiotics have against bacteria?
- Antibiotics can be categorised based upon whether they kill or inhibit bacterial growth in vitro
- Whilst this concept is simple the true definitions are more complex as most antibiotics kill bacteria at higher concentrations, and are therefore based on the ratios between the minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC)
- Cause bacterial death whilst the host cells remain undamaged
- Defined by a ratio of minimum bacteriocidal concentration (MBC) to minimum inhibitory concentration (MIC) of ≤ 4
- Limit bacterial growth by inhibiting ribosomes and DNA replication, with clearance of the cells by an intact immune system
- In high concentrations do cause bacterial death
- Defined by a ratio of minimum bactericidal concentration (MBC) to minimum inhibitory concentration (MIC) of >4
Which antibiotics are bacteriocidal and which are bacteriostatic?
V ancomycin
F luoroquinolones
P enicillin
A minoglycosides*
C ephalosporins
M etronidazole*
E rythromycin (macrolides)
C lindamycin
S ulfamethoxazole
T rimethoprim
T etracycline
C hloramphenicol
What is the minimum inhibitory concentration (MIC) and the minimum bactericidal concentration (MBC)?
Why are these concentrations useful?
Of use when:
• Detecting resistance
• Determining dosing schedules in antimicrobials with complex pharmacokinetics
• When looking at new antimicrobials
In regards to antibiotics, what are time-dependent and concentration-dependent killing?
- Refers to agents whose activity depends on the amount of time the serum concentration is above the minimum inhibitory concentration (MIC)
- They are therefore dosed regularly to keep the serum levels above MIC as long as possible
- Their half-life may be increased by the addition of a drug that reduces elimination of the antibiotic, e.g. probenecid given in penicillin treatment
- Infusions may also be useful
- B-lactams
- Erythromycin
- Clindamycin
- Linezolid
- Vancomycin
- Refers to agents whose activity correlates with peak serum concentration and the duration of exposure that bacteria have to the drug is less important to the killing process
- Usually exhibit significant 'postantibiotic effect' - the persistent suppression of bacterial growth after a brief exposure to the antibiotic, long after the serum concentration has fallen below the MIC
- High doses are used, with lower frequency of administration
- Aminoglycosides
- Quinolones
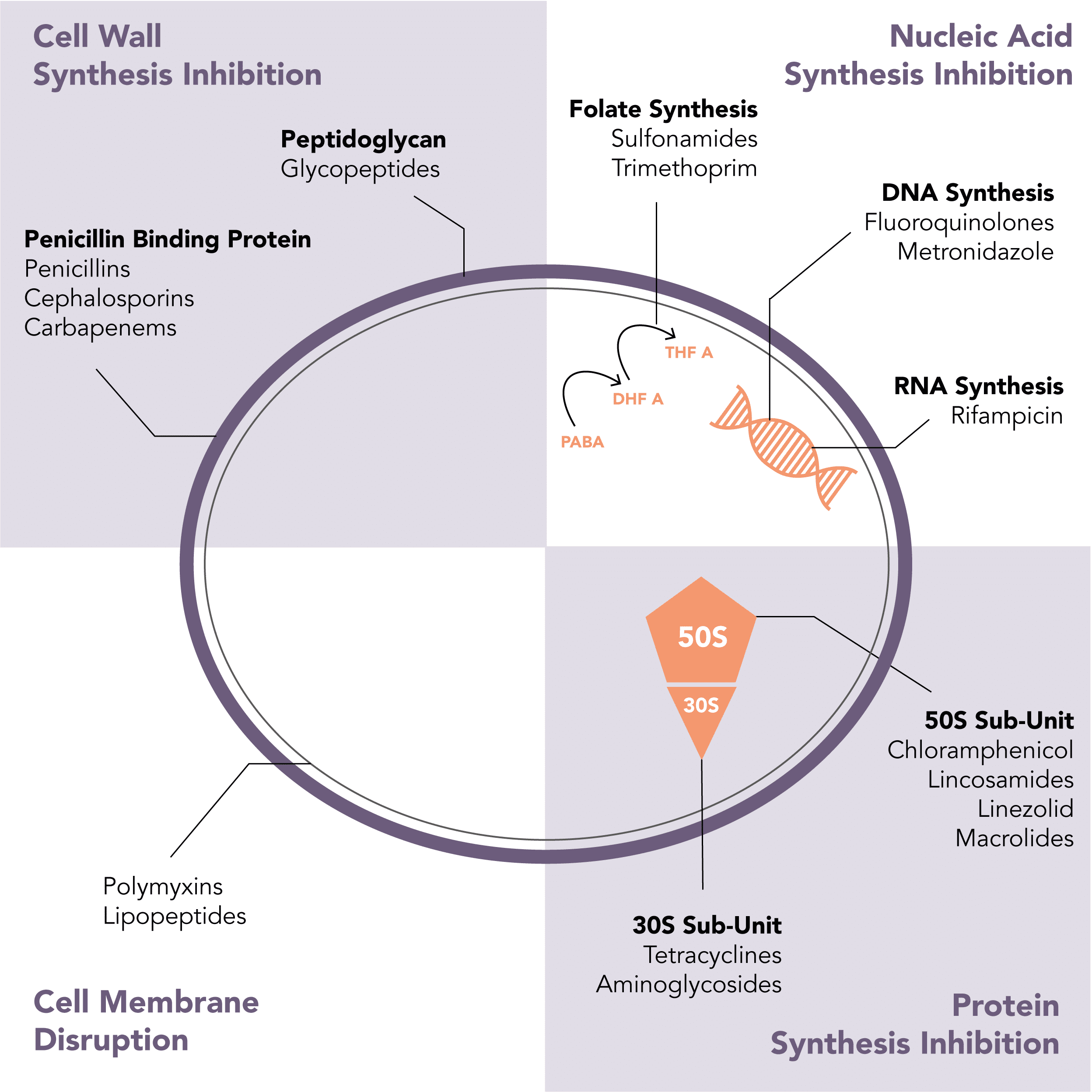
Mechanism of Action
What is the mechanism of action of antibiotics?
- Penicillins
- Cephalosporins
- Carbapenems
- Glycopeptides
- Polymyxins
- Lipopeptides
Can be remembered using the mnemonic CLEAN-TAG
- Chloramphenicol
- Clindamycin (Lincosamides)
- Linezolid
- Erythromycin (Macrolides)
- Tetracyclines
- Aminoglycosides
- Fluoroquinolones
- Metronidazole
- Rifampicin
- Sulfonamides
- Trimethoprim
Spectrum of Activity
How can the spectrum of activity of an antibiotic be described?
- Every antibiotic is characterized by a natural spectrum of antimicrobial activity:
- Includes the strains that can be inhibited by the antibiotic
- The number species within each strain that are susceptible may vary over time with the emergence of resistance
- Activity is often described as being either narrow-spectrum or broad-spectrum:
- No clear consensus definitions of these terms
- Often considered in terms of the effect on gram-positive and negative bacteria
Which bacteria do antibiotics have activity against?
A guide to the spectrum of activity of selected antibiotics has been produced as part of the excellent Wellington ICU Drug Manual
Initiation of Therapy
What are the principles of initiating antibiotic therapy?
- Determine the need for antibiotics:
- Once the decision is made to commence antibiotics, they should be administered without delay
- In stable clinical situations a ‘watch and wait’ strategy may be used
- Ensure appropriate microbiological sampling before commencing antibiotics
- Blood cultures should be obtained from venepuncture rather than indwelling arterial or venous catheters
- Ideally two separate sets of cultures should be taken
- Blood cultures should be taken from a venepuncture site, after adequate skin antisepsis, and not
- Ensure an appropriate antibiotic therapy is selected – usually based on an initial ‘best guess’ of the causative organism dependent upon the source:
- Empirical therapy must take into local resistance patterns and the likelihood of a resistant organism as assessed by:
- Recent courses of antibiotics
- LOS in hospital/ICU
- Whether they were admitted from home or another institution
- Choice should be guided by local antibiotic guidelines and specialist microbiology input is mandated in the case of serious infections
- Combination therapy is often used initially until culture results are back in severe infections
- Empirical therapy must take into local resistance patterns and the likelihood of a resistant organism as assessed by:
- Monitor for serum levels of potentially toxic antibiotics, particularly in those with hepatic or renal dysfunction
When should you start antibiotics on the ICU and in critical illness?
The timing of initial therapy should be guided by the urgency of the situation
- In critically ill patients therapy should be started immediately:
- Surviving Sepsis Campaign guidelines recommend that antibiotics be initiated within 1 hour from recognition of sepsis
- Immediate therapy also indicated in severe infections such as febrile neutropenic patients and patients with bacterial meningitis
- Therapy should be initiated immediately after or concurrently with collection of diagnostic specimens
- In more stable clinical circumstances should be deliberately withheld whilst confirming diagnosis or until appropriate specimens have been collected:
- Can avoid unnecessary antiobiotic exposure and related harm
- Can help patients receive appropriate therapy in the ICU where diagnosing infection is frequently challenging:
- Non-infectious conditions frequently mimic infection (e.g. pyrexia, tachycardia, leucocytosis)
- Premature antibiotic administration can limit yield in conditions where Appropriate in a microbiological diagnosis is critical to management, with long term targeted antibiotic therapy required to achieve cure
- If this approach is taken:
- Source control should not be delayed
- Close monitoring for signs of deterioration in a high dependency area should occur
- Examples where may be appropriate include:
- Patient with undifferentiated fever
- Patient with a colonised catheter
- Whilst awaiting specimens in endocarditis (Multiple sets of blood cultures) or osteomyelitis/diskitis (Disk space aspirate and/or bone biopsy)
Denny et al have offered guidance on situations where each strategy may be appropriate:
When should combination antibiotic therapy be used?
- Combination therapy is used in this setting to ensure that at least one of the administered antimicrobial agents will be active against the suspected organism
- Particularly important in for health care–associated infections where antibiotic resistance is common to multiple antibiotics
- Some antibiotic combinations act synergistically and with effects greater than the sum of their independent activities
- Combination therapy should be used when this is of proven clinical benefit
- An example is the combination of certain b-lactams and aminoglycosides which exhibits synergistic activity against a variety of gram-positive and gram-negative bacteria
- If infections are thought to be caused by more than one organism, a combination regimen may be used to extend the antimicrobial spectrum to cover all likely bacteria
- An example is intra-abdominal infections which are usually caused by multiple organisms with a variety of gram-positive cocci, gram-negative bacilli, and anaerobes
- Certain organisms are prone to developing resistance particularly in the setting of prolonged antibiotic treatment duration
- Combination of antimicrobial agents with different resistance to mechanisms of action can reduce the chance of a resistant strain being selected out leading to therapy failure
- An example is the use of combination therapy in the treatment of tuberculosis
Which factors influence antibiotic choice in critically ill patients?
- Allergy status
- Underlying organ function:
- Renal failure
- Liver failure
- Pregnancy and lactation
- Risk of side effects including C. difficile infection
- Potential drug interactions with current therapies
- Risk factors for acquiring drug resistant organisms:
- Recent courses of antibiotics
- LOS in hospital/ICU
- Residence in a nursing home or healthcare institution
- Recent travel
- Occupation
- Likely site of infection and causative organism(s)
- Severity of infection
- Broad spectrum antibiotics in fulminant infection
- Clindamycin in Group A streptococcal infection
- Risk of atypical infections
- Intravenous drug use
- Immunodeficiency
- Risk of atypical infections
- Microbiological data:
- Diagnostic culture results
- Results from screening (e.g. MRSA and VRE surveillance)
- Penetration to target tissue (e.g blood-brain barrier penetration in meningitis)
- Toxicity
- Cost
- Availability
De-escalation of Therapy
What is antibiotic de-escalation?
No clear consensus definition but general agreement that de-escalation involves:
- Changing an antimicrobial for another molecule with a narrower spectrum of activity
- Stopping one or more components of combination therapy
What is used to guide antibiotic de-escalation decisions?
- De-escalation decisions are guided by clinical and microbiological data:
- Typically reviewed at day 3 when results should be available and clinical response should be observed with appropriate treatment
- Clinical response following antibiotic initiation:
- Assessment of standard observations such as pulse, temperature, blood pressure, and oxygen saturation
- Clinical and radiological examination
- Consider other non-infectious causes for the patient’s condition
- Microbiological culture results:
- If positive, sensitivities can guide choice when narrowing the spectrum of antibiotic activity
- If negative, clinical risk assessment made on whether to continue, de-escalate or stop antibiotics
Has de-escalation proved to be beneficial?
- Evidence for de-escalation has in general shown comparable outcomes at worse and improved patient outcomes at best
- A large meta-analysis of 19 studies showed:
- No significant difference in adjusted mortality with de-escalation for infections of all type (adjusted OR 0.83, 95% CI 0.59-1.16)
- Significant unadjusted association between de-escalation and survival in bacteraemia/severe sepsis (OR 0.45, 95% CI 0.30-0.67)
- Rationale for potential benefit is proposed to be due to the detrimental effect of longer-term broad-spectrum antibiotics and the development of resistant organisms
- Generally felt evidence support the implementation of de-escalation and that it delivers at least comparable clinical outcomes to a conventional approach
- Despite this, rates of de-escalation range from about 10%-70% in studies of clinical practice
What are the principles of antibiotic de-escalation?
- Every patient with severe sepsis on antibiotic therapy should have the need for this considered and formally documented every day
- No later than day 3, a full assessment of investigation results and clinical progress should be performed and a positive decision should be captured to:
- Stop the treatment
- Narrow the spectrum of the therapy
- Reduce the number of antibiotics being used
- Not to de-escalate
- Every day thereafter a positive decision to stop, change, or continue the therapy should be made against specific reasons
- At every assessment the goal is to stop the therapy, or elements of the therapy, unless a positive and persuasive need for their continuation exists
Cessation of Therapy
How do you determine the optimal duration of antibiotics?
- Optimal duration means prescribing the selected drug for the shortest amount of time required for clinical and microbiologic efficacy
- Must balance efficacy (clinical and/or microbiological) and risk of adverse events (tolerability, relapses, emergence and spread of resistance)
- Varies considerably based on the severity and site of infection
- Given the potential for deleterious effects of prolonged courses recent emphasis has been on prescribing shorter courses
- There are several ways the duration of the antibiotic course can be determined:
- The traditional approach involves giving antibiotic therapy for a fairly arbitrary number of days based mostly on expert opinion rather than evidence:
- e.g. previous recommendations suggested 14 days to treat pyelonephritis, 10-14 days for community acquired pneumonia and 21-28 for bloodstream infections
- Main aim of treatment to minimise failure and avoid undertreatment
- Still used for infections that are difficult to treat such as bacterial endocarditis or tuberculosis where there is evidence to support prolonged courses
- Recent evidence supports shorter courses as effective in most infections:
- e.g. 3 days to treat uncomplicated UTI in females
- Desire to reduce effective duration of antibiotics has led to the increased use of biomarkers to help determine resolution of infection
- C-reactive protein (CRP) and procalcitonin (PCT) shown to be the most useful
- Evidence suggests can shorten antibiotic courses by about one day in sepsis
- Relies on having serial measures of infection biomarkers throughout the illness
- Should be used with clinical judgement taking in to account clinical and radiological signs of resolution or worsening of infection
How can procalcitonin (PCT) be used to guide the cessation of antibiotics?
An example protocol from the PRORATA trial demonstrates how PCT levels can be used to help guide decisions to stop antibiotics:
Monitoring Therapy & Response
Why do we monitor antibiotic levels? Which antibiotic levels in common use are monitored routinely and why?
Antibiotic levels are monitored:
- To ensure efficacy – usually for concentration-dependent antibiotics
- To prevent toxicity – drugs with a narrow therapeutic index (often low volume of distribution)
- Efficacy
- Toxicity:
- Ototoxicity and nephrotoxicity
- Toxicity associated high trough levels
- Easy to get high levels with organ failure especially renal failure
- Efficacy:
- Needs to maintain an effective level in the blood for a period of time to have its bactericidal effect on bacterial cell walls
- Levels may be even more difficult to judge when patient on CVVH
- Toxicity:
- Ototoxicity and nephrotoxicity
How do you monitor response to antibiotic treatment?
- Clinical parameters:
- Resolution of fever
- Improving tachycardia or confusion
- Laboratory values:
- Decreasing leukocyte count or CRP
- Radiologic findings:
- e.g. Decrease in the size of an abscess or degree of consolidation
- Caution should be applied as radiological improvement can frequently lag behind true clinical improvement
- Resolution of bacteraemia:
- Clearance of the bloodstream is as important as clinical improvement
- Persistent bacteraemia can represent an inadequately treated source or to the existence or development of endovascular infection (e.g. endocarditis or an intravascular device infection) - may be the only clue to this
What are the causes of early antibiotic failure?
- Wrong antibiotic choice
- Delayed administration of antibiotics
- Inadequate antimicrobial blood levels
- Inadequate penetration of the antimicrobial to the target site
- Inadequate source control
- Antimicrobial neutralization or antagonism
- Superinfection or unsuspected secondary bacterial infection
- Non-bacterial infection
- Non-infectious source of illness
Overview
When should antibiotic prophylaxis be given? What are the benefits and risks?
- Must only be given when evidence of benefit clearly outweighs the risks associated
- In general, used in situations where:
- Infection is rare but carries a high morbidity and mortality rate
- Infection is common, but not fatal
- Determination of benefits is often difficult as there is not always robust evidence of evidence in specific situations
- Reduction in surgical-site infections
- Reduction in infection following high risk exposure
- Selection of resistant microorganisms and the induction of antibiotic resistance
- Antibiotic allergy and toxicity
- Risk of superinfection
- Alteration of bowel flora and C. difficile infection
What are the principles of antibiotic prophylaxis?
- The risk and benefit ratios must be acceptable
- The antibiotic must be in the blood or target tissue before the onset of the surgery or bacteraemia
- An antibiotic loading dose must be employed for high blood/tissue concentrations
- The antibiotic must be chosen on the basis of the single most likely microorganism to cause the infection or bacteraemia
- The antibiotic should be continued only as long as the microbial contamination of or from the operative site continues
What are the indications for antibiotic prophylaxis?
- Clean surgery involving the placement of a prosthesis or implant
- Clean-contaminated surgery
- Contaminated surgery
- Surgery on a dirty or infected wound
- Dental surgery in specific sub-groups of patients at risk for infective endocarditis
- Following contact with high-risk disease (e.g. Meningococcal disease)
- Post-splenectomy
- Following high risk animal bites
- Following upper GI bleeding in cirrhosis (prevention of SBP)
- Secondary prevention of rheumatic heart disease