RESOURCES
OBJECTIVES & QUESTIONS
Introduction & Definitions
What is the definition of antimicrobial resistance?
WHO Definition
The ability of a microorganism (like bacteria, viruses, and some parasites) to stop an antimicrobial (such as antibiotics, antivirals and antimalarials) from working against it.
NICE Definition
Loss of effectiveness of any anti-infective medicine, including antiviral, antifungal, antibacterial and antiparasitic medicines
What is the definition of multidrug-resistant (MDR), extensively drug-resistant (XDR) and pan-drug-resistant (PDR)?
Mechanisms and Development of Resistance
What is intrinsic and acquired antibiotic resistance?
Which mechanisms confer antibiotic resistance to bacteria?
- Lack of molecular target
- Lack of transport mechanism required for the antibiotic to enter the cell
- Membrane impermeability
- Lack of molecular target
- Lack of transport mechanism required for the antibiotic to enter the cell
- Membrane impermeability
- Beta-lactamase
- Aminoglycoside modifying enzymes (AMEs)
- Tetracycline resistance
- Beta-lactam resistance
- Quinolone resistance
- Tetracycline resistance
- Quinolone resistance
- Rifampin resistance
- Macrolide resistance
- Methicillin resistance in s. aureus due to the acquisition of an exogenous PBP (PBP2a)
- Vancomycin resistance in enterococcus
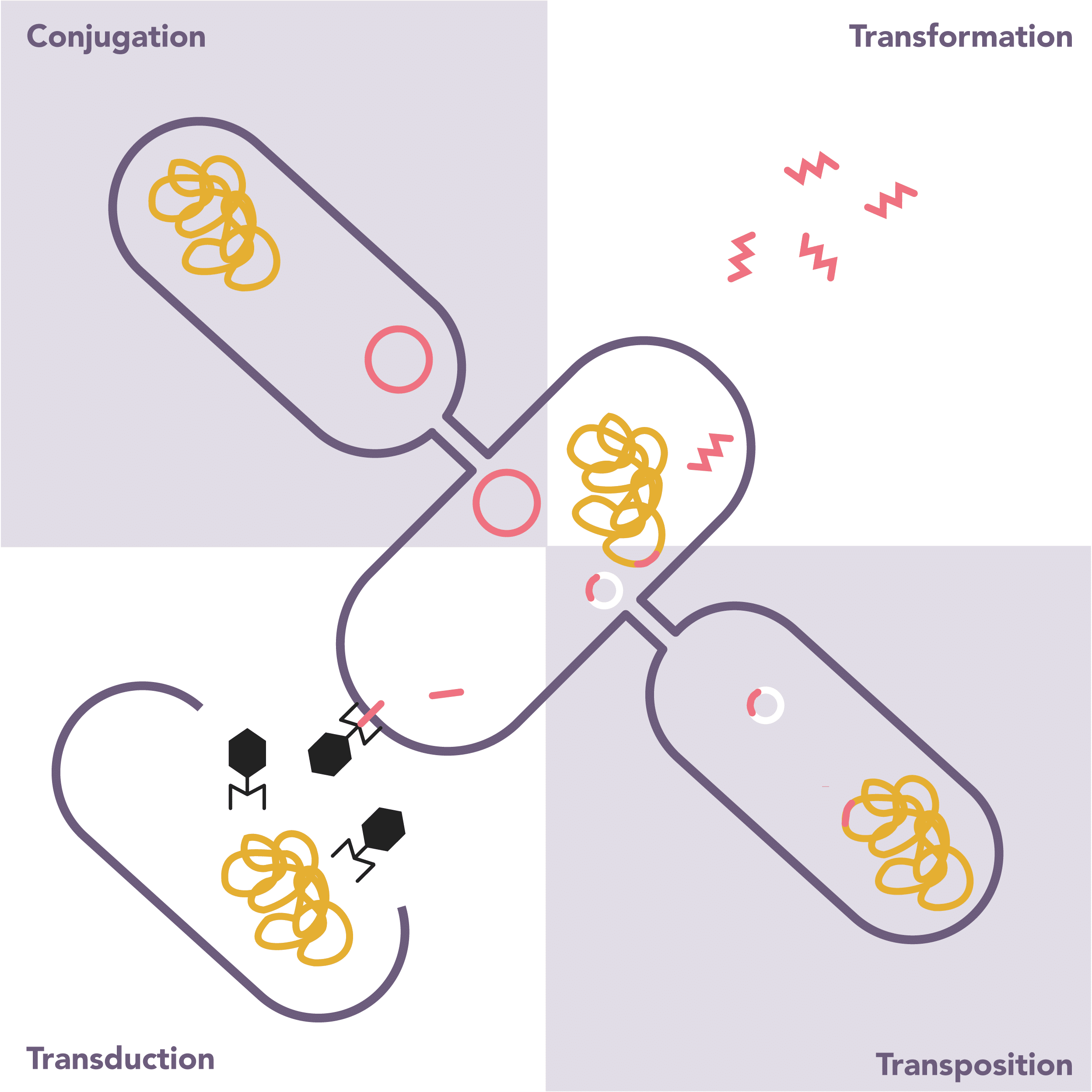
How do bacteria develop resistance to antibiotics?
- If no adverse effect on bacteria will be passed on to progeny
- Free DNA released from lysed bacteria
- Bacteriophages (viruses that infect bacteria) can transfer DNA from one bacteria to another
- Transfer of material through the direct contact of two bacteria, generally through plasmids
- Transposons (small segments of bacterial DNA) can move independently between plasmids or bacterial chromosomes
Epidemiology
How common is antibiotic resistance in the UK?
- Antibiotic resistance is an increasing problem in the UK
- Data is published annually by Public Health England in their report ‘English Surveillance Programme for Antimicrobial Utilisation and Resistance (ESPAUR)’
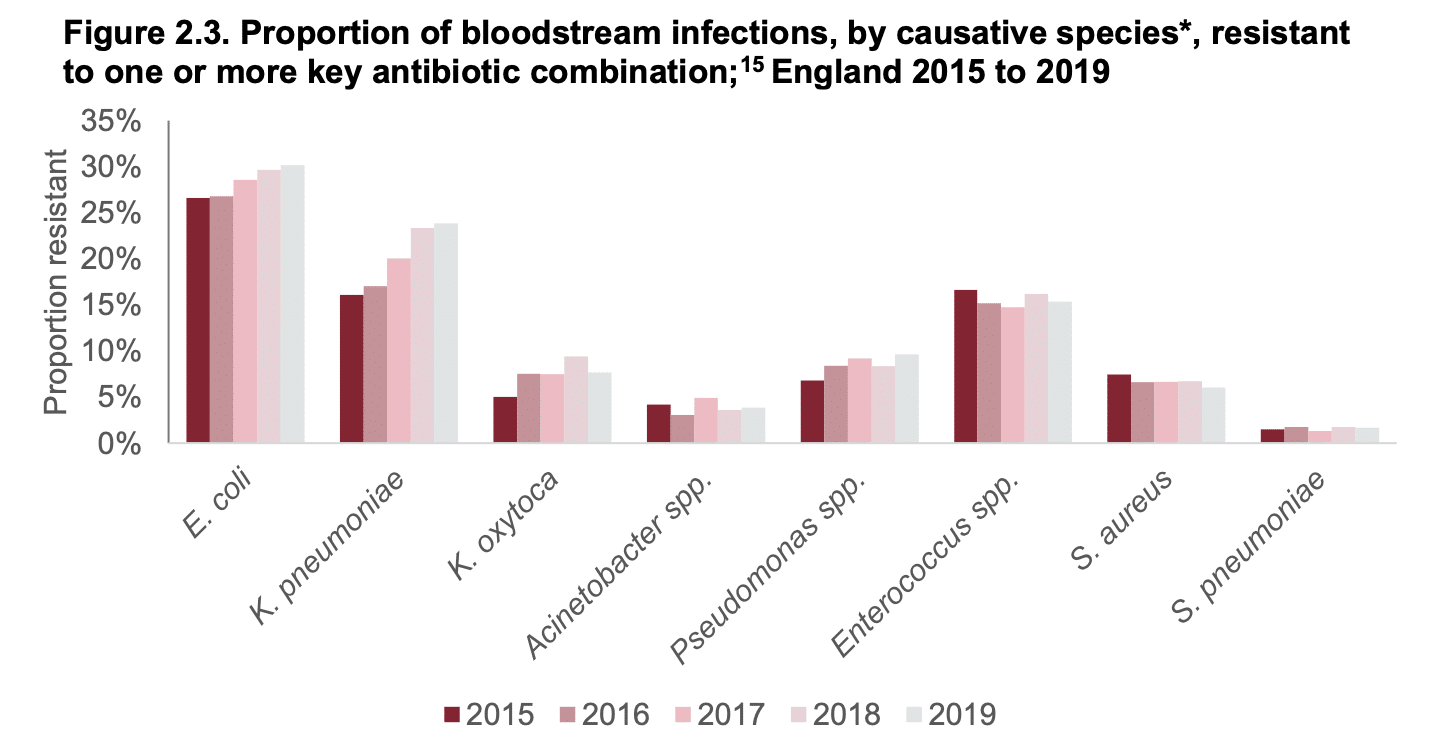
- Between 2015 to 2019, there was a 32% rise in bloodstream infections caused by bacteria resistant to one or more key antibiotics:
- The proportion of resistant isolates is particularly rising in E.coli, Klebsiella and Pseudomonas species:
Why is antibiotic resistance particularly common in the ICU?
- High numbers or antibiotics used (up to 70% of patient at any time
- Often broad spectrum
- Often prolonged use
- Repeated exposure
- Immunocompromised patients (due to therapy or comorbidities)
- Invasive devices breaching normal defences
- Area of high intensity care with potential for cross-contamination in a busy unit
Outcomes and Prognosis
What are the clinical consequences of infections with resistant organisms?
Infection with resistant organisms is associated with:
- Increased hospital length of stay
- Extra eight to twelve days for MRSA bacteremia
- Extra eighteen days for VRE bacteremia
- Increased treatment cost
- Up to £7,000 for MRSA bacteremia
- Up to £13,000 for VRE bacteremia
- Increased mortality
- Odds ratio of 2 for MRSA bacteremia
- Odds ratio of 2-5 for VRE bacteremia
- Odds ratio of 3.3 for CRO bacteraemia
Treatment Options
What are the treatment options for MDR gram positives?
- Active against MRSA and vancomycin-sensitive enterococci
- Vancomycin-intermediate S. aureus (VISA) isolates have been found clinically, and fear remains concerning vancomycin-resistant S. aureus (VRSA) isolates
- First of a new class of antibiotics called lipopeptides that interfere with gram-positive bacterial cell membrane function
- It has activity against MRSA, some VISA, and VRE
- May cause myositis, and creatine phosphokinase levels need to be monitored
- Inactivated by pulmonary surfactant and should not be used to treat pneumonia
- First oxazolidinone antibiotic
- Interferes with bacterial protein synthesis by binding to the 50S ribosome
- Has activity against MRSA and VRE
- Can be used orally or intravenously
- Can cause thrombocytopenia
- A combination streptogramin antibiotic that interferes with the bacterial 50S ribosome
- It has activity against MRSA
- Although it does not have activity against Enterococcus faecalis, it does work against Enterococcus faecium including VRE
- A new cephalosporin with activity against MRSA and penicillin-resistant S. pneumoniae
- As with all cephalosporins, there is no enterococcal activity including VRE
- A new lipoglycopeptide antibiotic that inhibits cell wall synthesis and disrupts bacterial cell membrane function
- It has activity against MRSA and enterococci but not VRE
- Doxycycline may have activity against both MRSA and VRE
- Trimethoprim-sulfamethoxazole (TMP-SMX) may have activity against MRSA
- Clindamycin may have MRSA activity, but it is important to check for erythromycin resistance because that may predict inducible clindamycin resistance
What are the treatment options for MDR gram negatives?
- Should remain active against most ESBL and Amp C-producing bacteria
- Rates of P. aeruginosa, Acinetobacter, and S. maltophilia resistance are increasing
- Binds to the lipopolysaccharides of the GNR outer membrane to disrupt it and cause cell death
- It has been used successfully intravenously and as an aerosol to treat MDR Acinetobacter
- It may be used for MDR P. aeruginosa and carbapenemase-producing Enterobacteriaceae
- Serratia, Proteus, and S. maltophilia are resistant
- Nephrotoxicity and neurotoxicity are the major toxicities.
- Parenteral derivative of minocycline
- May have activity against ESBL and carbapenemase-producing Enterobacteriaceae, S. maltophilia, and Acinetobacter
- P. aeruginosa and Proteus are resistant
- Because of low urinary concentrations, it should not be used to treat urinary tract infections
Overview
Which are the particular antibiotic-resistant organisms that are of concern in the ICU?
Methycillin resistant staphylococcus Aureus
- Resistant to: penicillins and cephalosporins
- Often resistant to clindamycin and quinolones in addition
- Important nosocomial pathogen which can cause penumonia, catheter and bloodstream infections
- Vancomycin / Teicoplanin
- Linezolid
- Rifampicin
Vancomycin resistant enterococcus
- Resistant to vancomycin and other glycopeptides
- As with other enterococci have intrinsic resistance to most penicillins and cephalosporins
- Can be a normal bowel flora but increasingly associates with nosocomial infections causing UTI, bloodstream or catheter infections and endocarditis.
- Linezolid
- Daptomycin
- Tigecycline
- Telavancin
- Quinupristin-dalfopristin
Form of β-lactamase
- Often inducible in ESCAPPM Organisms
- Provides resistance to penicillins and third generation cephalosporins
- Carbapenems
- Fluoroquinolones
- Aminoglycosides
- Cefepime (4th gen cephalosporin
- Temocillin
Extended-spectrum β-lactamases
- Often found in Klebsiella, Escherichia coli, and Proteus species
- Resistant to penicillins, 1st-3rd generation cephalosporins and monobactams
- Carbapenems
- Cefepime (4th gen cephalosporin)
Carbapenem resistant organisms
- Resistant to a broad-range of β-lactam antibiotics including carbapenems
- Tigecycline
- Colistin
- Significant ability to develop resistance particularly through inducible β-lactamases
- Marked ability to colonize indwelling lines, catheters and endotracheal tubes
- Fluoroquinolones
- Aminoglycosides
- Carbapenems
- Colistin
- Low virulence organism but inherently resistant to a wide range of antibiotics
- Isolation usually represents colonisation
- But in the setting of risk factors (immunosuppression, prosthetic material) can cause infection
- Colistin
- Tigecycline
MRSA
What is MRSA?
- A strain of Staphylococcus aureus that is resistant to all β- lactams antibiotics:
- Healthcare-acquired strains of MRSA are also MDR, being resistant to other classes of antibiotics such as macrolides and quinolones
- Often carries the virulence factor Panton-Valentine leukocidin (PVL)
- Causes mainly skin and soft tissue infection
- Associated with severe forms of necrotising pneumonia
How does MRSA confer its resistance?
- Penicillin-binding protein (PBP) is a form of transpeptidase required for the formation of the bacterial cell wall through cross-bridging of peptidoglycan unit. It is usually found in 4 forms (PBP1-4)
- Methicillin and other β-lactam antibiotics act by inhibiting PBPs, preventing cell wall formation
- MRSA expresses a foreign form of PBP2, known as P2Pa. This is not inhibited by β-lactam antibiotics, allowing cell wall formation to continue even in their presence.
What are the treatment options for MRSA?
- Generally considered first line therapy
- Effective therapy and may be beneficial for severe disease caused by PVL and exotoxins
- Expensive and associated with significant side effects
- Some strains show inducible resistance to clindamycin
- May be beneficial for severe disease caused by PVL and exotoxins
- May be used as initial therapy in non-severe infections
- May be used as initial therapy in non-severe infections
- Not used as monotherapy
- May be beneficial adjunct in severe infections
VRE
What is VRE?
- Vancomycin-resistant enterococci (VRE) are bacterial strains of the genus Enterococcus (faecium and faecalis)
- They are resistant to vancomycin and other glycopeptide antibiotics
- Also have high levels of resistance to aminoglycosides and ampicillin
- As with other enterococci, can reside in the gut forming a frequent source of colonisation
- Increasingly associated with nosocomial infections: catheter-related bloodstream infections, wound infections, UTIs and bacteraemia
How does VRE confer its resistance?
- Vancomycin acts by binding to glycopeptide cell wall precursors ending in D-Ala-D-Ala, inhibiting cross-linking and the production of the cell wall
- VRE have genes which produce glycopeptide chains with different terminal amino acids:
- Generated precursors ending in D-Ala-D-Lac, D-Ala, or D-Ala-D-Ser
- Have a low affinity for vancomycin preventing binding and inhibition
- Resistance may be chromosomally or plasmid-mediated
What are the treatment options for VRE?
- Tigecycline
- Linezolid
- Daptomycin
- Quinupristin-dalfopristin
- Fosfomycin
β-lactamases, ESBL & CRO
What are β-lactamases and how do they cause antibiotic resistance?
- β-lactamases are hydrolytic enzymes which cleave the β-lactam ring inhibiting the effect of β-lactam antibiotics such as penicillins and cephalosporins
- Bind covalently to the beta-lactam ring
- Act as proteases dissociating the –CO–NH structure of the ring
- The enzymes may be:
- Carried on bacterial chromosomes (inherent to the organism)
- Plasmid-mediated with the potential to move between bacterial populations
What are the different forms of β-lactamase?
- β-lactamases have been classified based on the molecular structure in a classification devised by Ambler
- Grouped into four classes according to the structure of the primary motif:
- Classes A, C, and D use a serine as an enzyme active centre
- Class B use the metal zinc as an enzyme active centre
- Within each class a number of different forms of beta-lactamases exist, each expressing individual resistance patterns
- Classical narrow-spectrum: PSE, CARB
- ESBLs (Extended-spectrum β-lactamases): TEM, SHV, CTX-M
Class A Carbapenemases: GES, KPC, SME, IMI, SHV-38, SFC-1
MBL (Metallo-β-lactamases):
Subclass B1: IMP, VIM, SPM, IND NDM, DIM, GIM, SIM
Subclass B2 CphA, Sfh-1, ImiS
Subclass B3, AIM, CAU-1, GOB-1, FEZ-1
- AmpC
ESAC (Extended-spectrum AmpC)
- ESBLs (Extended-spectrum β-lactamases): OXA
CHDLS (Carbapenem-hydrolyzing class D β-lactamases)
Carbampenemase Activity
What are the ESCAPPM organisms?
- ESCAPPM is a mnemonic for the organisms with inducible beta-lactamase activity:
- Usually due to chromosomally mediated AmpC β-lactamase (resistant to penicillins and third generation cephalosporin)
also resistant to clavulanic acid - Enzyme production is normally held at low levels by repressor mechanisms
- In the presence of a beta-lactam antibiotic, this repression is lifted and enzyme production is greatly increased
- Usually due to chromosomally mediated AmpC β-lactamase (resistant to penicillins and third generation cephalosporin)
- The mnemonic stands for:
- Enterobacter sp.
- Serratia sp.
- Citrobacter sp.
- Aeromonas sp.
- Proteus sp.
- Providencia sp.
- Morganella morganii
What are extended-spectrum β-lactamases (ESBLs)?
- ESBLs describe a group of plasmid-mediated β-lactamases offering resistance to a wide spectrum of antibiotics
- Defined by the ability to hydrolyse penicillins, 1-3rd generation cephalosporins and monobactams
- They do not hydrolyse carbapenems and can be inhibited by β-lactamase inhibitors such as clavulanate
- Organisms that commonly carry ESBLs include:
- Klebsiella pneumoniae
- Escherichia coli
- Proteus mirabilis
- Enterobacter cloacae
- Non-typhoidal Salmonella (in some countries)
Overview
What is antibiotic stewardship?
An organizational or healthcare-system-wide approach to promoting and monitoring judicious use of antimicrobials to preserve their future effectiveness
What are the components of antimicrobial stewardship and what other aspects are important in preventing antibiotic resistance?
(Mainstay of Preventing Resistance)
- Establish an antimicrobial stewardship programme
- Providing education and training to health and social care practitioner
- Ongoing audit of antimicrobial practice
- Ensure that laboratory testing reported is in line with national and local guidelines
- Ensure evidence based antimicrobial guidelines in place
- Ensure pharmacy and microbiology involvement in antimicrobial decisions on the ICU
- Cycling of antibiotics within an organisation
- Rapid identification of infection
- Aim to obtain microbiological samples prior to initiating empirical antibiotic therapy whenever possible
- Institute antibiotics within 1 hour of decision:
- If non-severe infection consider awaiting samples before initiating
- Instigate appropriate broad spectrum therapy initially dependent on likely source
- Follow local (where available) or national guidelines
- Assess Likelihood of resistant organisms as assessed by:
- Recent courses of antibiotics
- LOS in hospital/ICU vWhether they were admitted from home or another institution
- Consider pharmacokinetic / pharmacodynamic properties
- Prescribe the most appropriate dose and route
- Use combination therapy for septic shock
- Review all treatments at 48-72 hours
- Check microbiology and determine appropriate therapy
- Consider de-escalation to targeted single therapy at earliest opportunity
- Consider switch to oral therapy
- Prescribe the shortest effective course
- Side rooms for patients with resistant organisms
- Screening and decolonisation (e.g. MRSA)
- Barrier nursing, signs
- Alcohol gel and hand washing
- Chlorhexidine bath
- Audit of IC measures
- Prevention bundles: SSI, VAP, CVC
- Development of novel antibiotics
- Alternative treatments
- Development of diagnostic tests