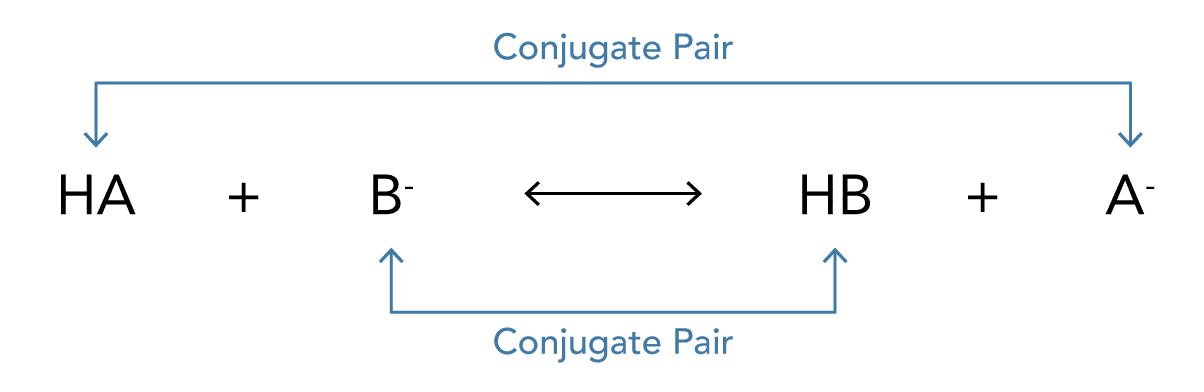
- The concept of conjugate acids and bases arise from the Bronsted-Lowry acid-base theory
- When an acid (HA) dissociates into its ions in a solvent it loses a hydrogen ion (H+)
- The species that is formed is the “conjugate” base of the acid (A–) – it is a base as it is able to absorb the hydrogen ion that has been lost
- The reaction can be summarised as:
- Examples of commonly encountered acids and their conjugate bases include:
Acid
(HA)
(HA)
Conjugate Base
(A-)
(A-)
Hydrochloric Acid
HCl
HCl
Chloride Ion
Cl-
Cl-
Carbonic Acid
H2CO3
H2CO3
Bicarbonate Ion
HCO3-
HCO3-
Lactic Acid
C3H6O3
C3H6O3
Lactate Ion
C3H5O3-
C3H5O3-