OBJECTIVES & QUESTIONS
Overview
How can you classify non-steroidal anti-inflammatory drugs (NSAIDs)?
Group
Class
Drug
Specific COX-2 Inhibitors
Salicylates
- Aspirin
Specific COX-2 Inhibitors
Acetic acid derivatives
- Diclofenac
- Ketorolac
- Indomethacin
Specific COX-2 Inhibitors
Anthralinic acids
- Mefanamic acid
Specific COX-2 Inhibitors
Propionic acids
- Ibuprofen
- Naproxen
Specific COX-2 Inhibitors
Para-aminophenols
- Paracetamol
Preferential COX-2 Inhibitors
Oxicams
- Tenoxicam
- Piroxicam
Specific COX-2 Inhibitors
Pyrazoles
- Parecoxib
- Celecoxib
Specific COX-2 Inhibitors
Methylsulfones
- Etoricoxib
Pharmacokinetics
What are the classical pharmacokinetics of NSAIDs?
Absorption
- Most are well absorbed through the small bowel and have high bioavailability
- Antacids may delay absorption
- Diclofenac undergoes first pass metabolism reducing bioavailability and is often given rectally or intravenously
Distribution
- Highly protein bound (mostly to albumin) - around 99% in most cases
- Have a low apparent volume of distribution (<0.2 L/kg)
- Competition for protein binding sites can lead to enhanced action of other drugs as they are displaced (oral anticoagulants and hypoglycaemics)
- High protein binding means NSAIDs are not dialysable
Metabolism
- Primarily eliminated by metabolism in the liver
- Liver disease can significantly alter behaviour
Excretion
- Excreted in an inactive form in urine and bile
Mechanism of Action
What are prostaglandins?
- Biologically active lipid compounds derived from arachidonic acid
- Like hormones act as chemical messengers but act on the cell they are synthesized in rather than moving to other sites
- Variety of prostaglandins exist with different physiological effects:
PGD2
- Vasoconstriction
PGE2
- Vasodilation (particularly renal afferent vessels)
- Increase in gastric mucous and bicarbonate production
- Suppression of lymphocytes
- Sensitization of peripheral nerve ending to pain
- Involved in fever generation
PGF2
- Mixed vascular effects
- Uterine contraction and cervical ripening
PGI2
(Prostacyclin)
(Prostacyclin)
- Vasodilation (particularly renal afferent vessels)
- Inhibition of platelet aggregation
- Bronchodilatation
- Sensitization of peripheral nerve ending to pain
TXA2
(Thromboxane)
(Thromboxane)
- Vasoconstriction
- Induction of platelet aggregation
- Bronchoconstriction
- Uterine contraction
What is cyclooxygenase? What are the different forms and where are they found?
- Group of enzymes responsible for the production of prostanoids from arachidonic acid
- Prostanoids are biologically active molecules including prostaglandins and thromboxane
- Two primary isoforms of cyclooxygenase (COX) are described:
COX-1
(Constitutive Form)
(Constitutive Form)
- Located throughout most tissues in the body
- Plays a house keeping role in most tissues
- Activity most strongly associated with:
- Prostaglandin production in gastric mucosa - stimulate bicarbonate and protective mucus secretion
- Prostaglandin production in renal vasculature - promote vasodilatation of glomerular afferent vessels
- Thromboxane production in platelets - mediates platelet aggregation and adhesiveness
COX-2
(Inducible Form)
(Inducible Form)
- Usually absent in most tissues except brain, uterus and kidneys
- Up-regulated in other tissues in response to damage and inflammatory cytokines:
- Facilitates the inflammatory response at site of injury
- Mediates production of prostacyclin (PGI2) in vascular endothelium
- Third type called COX-3 has been described
- Splice variant of COX-1 enzyme
- Exists in central nervous system
- COX-3 inihibition is a proposed mechanism of paracetamol activity
What is the mechanism of action of NSAIDs?
- Inhibit the enzyme cyclo-oxygenase.
- Prevents the production of:
- Prostaglandins (including prostacyclins)
- Thromboxane
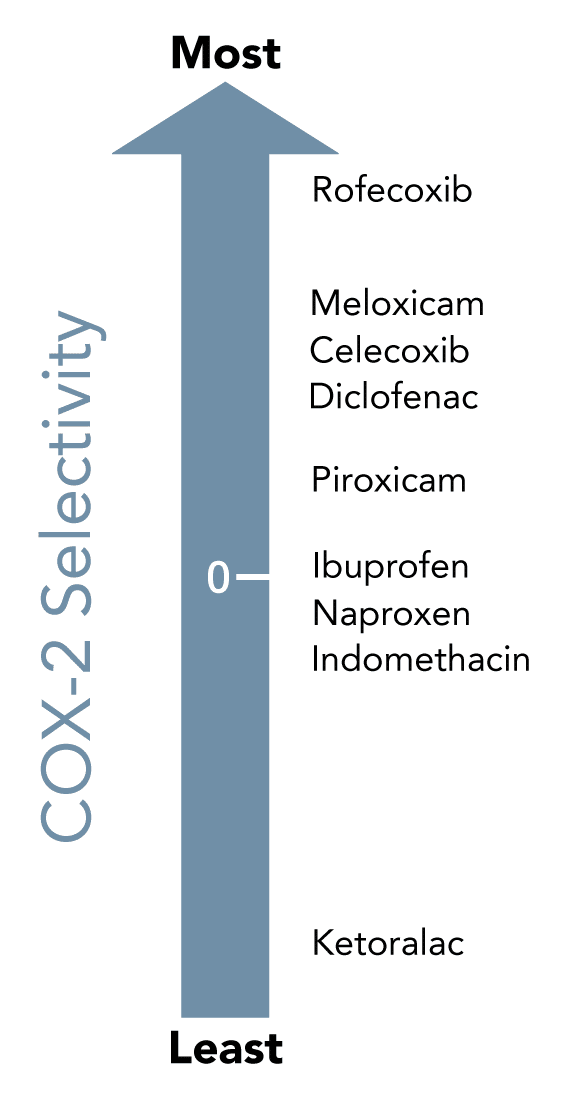
Which COX enzymes are inhibited by specific NSAIDs?
Side Effects & Contraindications
What are the clinically significant side effects of NSAIDs?
Gastrointestinal System
- Dyspepsia
- Gastroduodenal ulcers
- GI bleeding and perforation
- Hepatotoxicity (transaminitis seen in up to 15% after prolonged use)
Cardiovascular System
- Coronary thrombosis and myocardial infarction
- Hypertension
Renal System
- Reduce glomerular filtration rate (leading to AKI or CKD)
- Sodium retention
- Acute interstitial nephritis
Respiratory System
- Precipitation of bronchospasm in asthmatics
Haematological System
- Impaired platelet function
- Increased risk of bleeding
Pregnancy
- Interference with ovulation and implantation
- Premature closure of the patent ductus arteriosus
- Prolonged gestation and labour
What are the GI side effects of NSAIDs and why do they occur?
- When gastric mucosa damaged prostaglandins released:
- Stimulate secretion of protective mucous layer
- Stimulates bicarbonate ions to neutralise acid
- COX-1 inhibition reduces synthesises of prostaglandins:
- Prevents production of gastric protective elements
- Leads to gastric and intestinal erosions and ulcers
- Potential for GI bleeding increased due to effect on platelet function
What are the renal complications of NSAIDs and why do they occur?
- Renally produced prostaglandins essential for maintenance of adequate perfusion pressure:
- Cause vasodilatation of afferent vessels
- COX-1 expressed in renal vasculature and glomerular cells:
- Inhibition reduces synthesis of prostaglandins
- Prevents normal haemostatic mechanisms to maintain afferent flow, glomerular perfusion and filtration rate
- Precipitate sodium and fluid retention:
- Can exacerbate hypertension
- Average increase in MAP of 5-10mmHg with NSAIDs
- Long term NSAID use has been associated with interstitial nephritis
What are the cardiovascular complications associated with COX-2 inhibitors? What are the underlying mechanisms for this?
- Increased risk of coronary vascular events
- 3 extra coronary events per 1,000 patients per year
- Trend towards increased risk of stroke but not reached significance
- Has resulted in a number of high profile drug withdrawals including rofecoxib, lumiracoxib and etoricoxib
- European review concluded diclofenac has a similar thrombotic risk profile
- COX-2 inhibitors alter the delicate thromboxane/prostacyclin balance in favour of platelet aggregation, vasoconstriction and thromboembolism:
- Inhibit prostacyclin production mediated via COX-2
- Thromboxane production unaffected as mediated by COX-1
What are the haematological effects of NSAIDs and why do they occur?
- Thromboxane A2 plays a key role in platelet aggregation and activation
- COX-1 inhibition reduces synthesises of thromboxane A2 inhibiting the haemostatic process
- May increase blood loss in the perioperative period
- Effect is used therapeutically for prevention of thrombotic disease (aspirin)
What are the effects of NSAIDs on bone and why do these occur?
- COX-2 plays important role in bone healing with prostaglandins increasing cortical mass and trabeculation in animal models
- Evidence is equivocal as to whether NSAIDs prevent bone healing in humans:
- No strong evidence to preclude their use in fractures or orthopaedic surgery
Why are asthmatics often sensitive to NSAIDs?
- COX-2 inhibition increases the amount of arachidonic acid metabolised by lipooxygenase to leukotrienes
- Leukotrienes known to cause bronchospasm
- Middle aged asthmatics usually more affected than children
Diclofenac
What are the clinical properties of diclofenac?
Formulations
- Oral: 25/50/100 mg tablets
- Rectal: 12.5/25/50/100 mg suppositories
- IV / IM: clear colourless solution in ampules containing 25 mg/ml or 75 mg/3ml
Posology
- Oral: 75-150mg daily in 2-3 divided doses
- Rectal: 75-150mg daily in 2-3 divided doses
- IV / IM: 75mg, OD or BD for a maximum 2 days
Before administration of any drug confirm dosage in the BNF
Indications
- As per other NSAIDs
- IV and PR preparations lend itself to use as intraoperative analgesia
Contraindications
- As per other NSAIDs
Chemical Structure
- Acetic acid derivative
Absorption
- The drug is well absorbed when administered by all routes
- Oral bioavailability is 60% due to significant first-pass metabolism
Distribution
- 99.5% protein-bound in the plasma, predominantly to albumin
- The VD is 0.12–0.17 l/kg
Metabolism
- Principally metabolised in the liver
- Undergoes hydroxylation and methoxylation to phenolic metabolites
- Subsequent conjugation to inactive glucuronide and sulphate metabolites
Excretion
- Approximately 65% of the dose is excreted in the urine and 35% in the bile
- Less than 1% is excreted unchanged
Mechanism of Action
- As per other NSAIDS acts as a COX inhibitor
- In vitro selectivity for COX-2 inhibition:
- Lower than that of celecoxib,
- Higher than ibuprofen, and naproxen
Ibuprofen
What are the clinical properties of ibuprofen?
Formulations
- Oral: 50/100/200/400 mg tablets
Posology
Before administration of any drug confirm dosage in the BNF
- Oral: 300-400mg 3-4 time daily
- Max 600mg 4 times daily
Indications
- As per other NSAIDs
Contraindications
- As per other NSAIDs
Chemical Structure
- Propionic acid derivative
Absorption
- The drug is well absorbed
- Oral bioavailability is 80%
Distribution
- 90-99% protein-bound in the plasma, predominantly to albumin
- The VD is 0.14 l/kg
Metabolism
- Principally metabolised in the liver
- Undergoes oxidation to two inactive metabolites
Excretion
- Excreted in the urine
Mechanism of Action
- As per other NSAIDS acts as a COX inhibitor:
- Non-selective inhibitor (1:1 ratio for COX-1 and COX-2)