RESOURCES
Review Articles
- Tameem et al, BJA Ed (2013); Cerebral Physiology
- Das et al, Anaes Int Care (2019); Applied cerebral physiology
- Sakka et al, Eur Ann Otorhinolaryngology (2011); Anatomy and physiology of cerebrospinal fluid
- Shatzmiller et al, J Neur Dis & Stroke (2016); Blood-Brain Barrier Crossing for Therapeutic and Diagnostic Agents
OBJECTIVES & QUESTIONS
Fundamentals of ICP
- The pressure inside the skull and thus the pressure exerted on brain tissue
- Normal ICP in adults is 5-15mmHg
- It is constantly varying:
- Throughout the cardiac cycle
- Throughout the respiratory cycle
- With exercise, coughing and straining (can reach 50mmHg in the normal brain)
- Normal ICP is lower in children and can reach sub-atmospheric levels in neonates:
See sub-section: ICP Waveforms
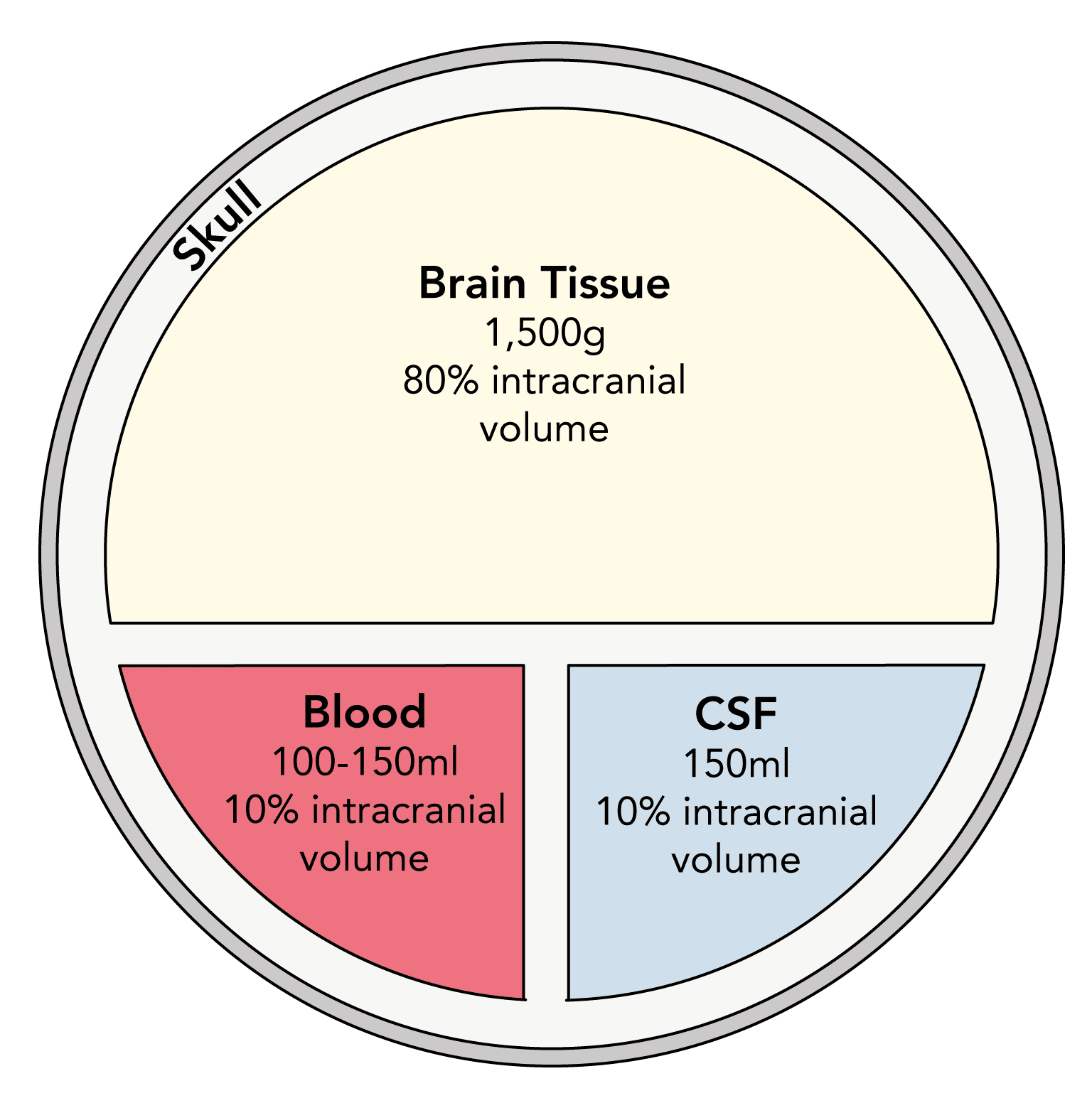
Determinants of ICP
The skull of an adult is in effect a rigid box which contains brain tissue, blood and CSF. An increase in the volume of one component invariably results in an increase in ICP unless the volume of another component decreases
- The relationship between ICP and intracranial volume can be demonstrated using the intracranial compliance curve
- Compliance reflects the ability to compensate for changes in volume without subsequent rises in ICP – with reducing compliance comes large rises in ICP
- The curve can be described in three stages:
(Initial stage)
- Characterised by high compliance and low ICP
- Compensatory mechanisms act to prevent rises in ICP associated with increased intracranial volume:
- CSF moves into the spinal subarachnoid space (primary mechanism)
- Blood displaces into extracranial vessels
- Brain itself has minimal compressibility therefore is very limited in scope for compensation
- Reserve volume varies with age due to ongoing cerebral atrophy:
- 60–80 mL in young persons and approximately 100–140 mL in geriatric population
(Transition stage)
- Characterised by low compliance and low ICP
- Gradual depletion of compensatory reserve as intracranial volume rises with slow increase in ICP
(Ascending stage)
- Characterised by low or null compliance and high ICP
- Compensatory mechanisms stop working and small changes in volume elicit high increases in pressure
- Results in a high risk of cerebral ischaemia and herniation
- As defined by the Monro-Kellie hypothesis an increase in the volume of any of the components inside the skull will invariably results in an increase in ICP unless compensation occurs
- Localised: tumour, abscess
- Generalised: oedema
- Hydrocephalus: congenital, acquired
- Haemorrhage: extradural, subdural or intraparenchymal
- Arterial: hypercapnia, hypoxia, seizures, increased metabolic rate
- Venous: impaired drainage, venous sinus thrombosis
See section: Raised ICP
See section: Intracranial Pressure (ICP) Monitoring
Fundamentals of CBF
How much oxygen does the brain consume?
- Resting oxygen consumption of the brain is ~50 ml/minute
- Consumes ~20% of total body oxygen requirements at rest
- Global CBF is ~50ml / 100g brain tissue / minute
- White matter: 20ml/100g/min
- Grey matter: 70ml/100g/min
- Receives ~15% of cardiac output at rest
- More sensitive to even short periods of reduced blood flow than other organs in the body
- Clinically a reduction in CBF to 30 ml/100g/min for as little as 5 seconds can result in syncope and loss of consciousness as occurs during a vasovagal episode
- Decreases in CBF lead to progressive cellular ischaemia:
- The net pressure gradient driving blood flow through the cerebral circulation which results in cerebral blood flow
- It depends upon the mean arterial pressure (MAP), the intracranial pressure (ICP) and the central venous pressure (CVP) – many exclude CVP from the equation as its effects are usually negligible:
CPP = MAP – (ICP + CVP)
- The normal CPP is 70–80 mmHg
- A CPP of 30-40 mmHg is the threshold for critical ischaemia
Factors Affecting CBF
- Changes in mean arterial pressure (MAP)
- Changes in intracranial pressure (ICP)
- Changes in central venous pressure (CVP)
- Myogenic control
- Metabolic mediators:
- pCO2
- pO2
- Cerebral metabolic rate
- Temperature
- pH
- Neurogenic mediators
- Endothelial mediators
- Chemical mediators:
- Volatile anaesthetics
- Intravenous anaesthetics
- Haematocrit
- According to the Hagen–Poiseuille equation blood flow increases as viscosity decreases
- Blood viscosity is determined by the haematocrit:
- High haematocrit (>50%) is associated with decreased viscosity and risk of intravascular sludging
- A lower haematocrit (<30%), whilst associated with enhanced flow, contains less haemoglobin and thus delivers less oxygen to brain parenchyma
- A balance must be struck between optimal rheology and oxygen delivery
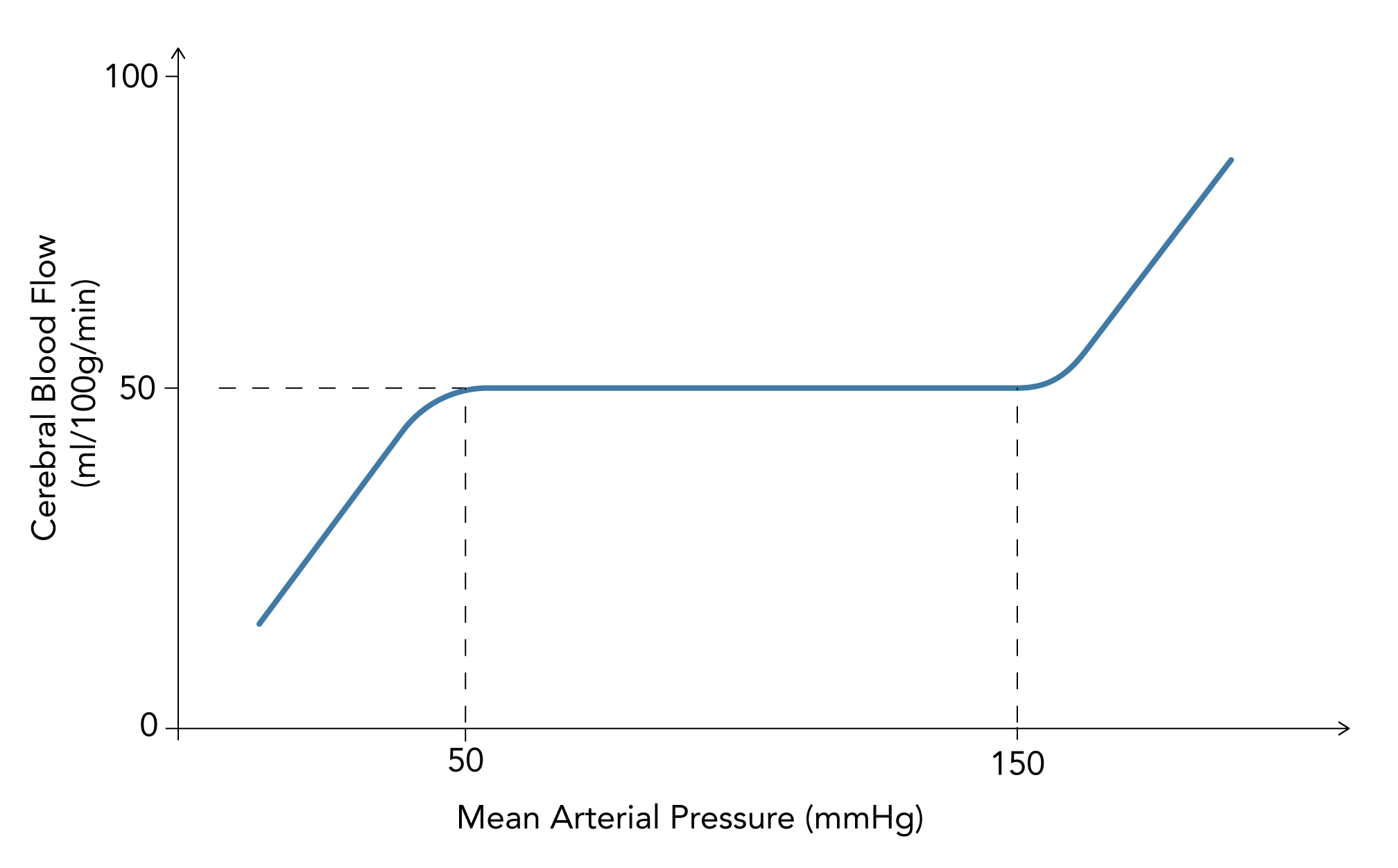
Autoregulation
The ability of an organ to regulate its blood flow despite changes in its perfusion pressure
- Cerebral blood flow is autoregulated between a MAP of 50 – 150 mmHg
- Outside the autoregulatory range:
- When MAP >150 mmHg CBF becomes directly proportional to CPP
- When MAP falls <50 mmHg CBF falls proportionally below the ‘normal’ value of 50 mL/100 g/min, resulting in ischaemia
- In hypertensive patients autoregulation occurs between a higher range of pressures (60 and 160 mmHg)
- Outside the autoregulatory range:
- Can be displayed using an autoregulation curve:
- Represents an oversimplification of the true relationship
- In vivo changes in perfusion pressure may be regional and there is not a neat linear relationship at each end of the curve
- Autoregulation becomes impaired in intracranial pathology (e.g. traumatic brain injury, infection and haemorrhage)
- Leads to a more directly proportional relationship between CPP and cerebral blood flow
- The autoregulatory vessel calibre changes are mediated by interplay between myogenic, neurogenic, metabolic and endothelial mechanisms:
- Thought to be the primary mechanism behind cerebral autoregulation
- Cerebral vascular smooth muscle vasoconstricts in response to increased wall tension and vasodilates in response to decreased wall tension
- Results in change of vessel calibre to maintain a constant cerebral blood flow
- Decreased perfusion due to a fall in perfusion pressure can lead to accumulation of metabolic products in tissue (H+/K+/adenosine/nitric oxide/CO2)
- Mediate cerebral vasodilatation and thus increased cerebral blood flow
- Important mechanism in smaller vessels that are subject to changes in the local environment
- Vascular smooth muscle is under autonomic control and mediates vasoreactivity in small and medium sized vessels
- Thought to play minor role in autoregulation in health
- Differences in regional innervation in the brain may contribute to the pathophysiology of certain conditions such as PRES
- Endothelial tissue secretes a number of vasodilators and vasoconstrictors in a paracrine manner
- Thought to play a minor role in cerebral autoregulation
Metabolic Mediators
- Increases linearly between a PaCO2 range of 3-10 kPa:
- Due to CO2 mediated vasodilatation
- Blood flow increases by 3-4% for each 0.13kPa (1mmHg) increase in partial pressure of carbon dioxide (PaCO2)
- Below 3.5 kPa, cerebral vasoconstriction leads to tissue hypoxia with subsequent reflex vasodilatation and maintenance of blood flow
- Above 10 kPa vasodilatation is maximal with no further increase in flow
- In chronic hypercapnia, the curve is shifted to the right, with a higher kPa over which linear vasodilatation occurs
- At ‘normal’ PaO2 of >8 kPa there is minimal effect on cerebral blood flow
- As PaO2 falls below this level there is a rapid rise in cerebral blood flow:
- Mediated by hypoxia-induced vasodilation
- Results in a CBF of around 110ml/100g/min at a PaO2 of 4.0 kPa
- May contribute to further rises in ICP in patients with head injury
- There is a linear correlation between cerebral blood flow and CMR
- Known as ‘flow–metabolism coupling’
- Exact mechanisms are unclear but are likely due to accumulation of vasodilatory metabolic by-products (e.g. CO2, H+, K+ and adenosine
- Occurs on a local level to match CBF to metabolically active areas of the brain:
- Demonstrated by the higher blood flow to the more metabolically active grey matter (70ml/100g/min) than white matter (20ml/100g/min)
- Also occurs on total brain level with CBF matched to total brain metabolism:
- Increase in overall CMR (during pyrexia or seizures) results in increased CBF
- Decreased CMR (during general anaesthesia or hypothermia) results in decreased CBF
Effect of Anaesthetic Drugs
(excluding ketamine)
- Propofol, thiopentone and etomidate all reduce CMR
- As a result of flow–metabolism coupling they all result in a fall in cerebral blood flow
- Autoregulation is not affected
- Causes increased cerebral blood flow
- Increases cardiac output and MAP
- Increases CMR and dilates cerebral vasculature
- Counteracts mild increase in ICP to maintain perfusion
(excluding N2O)
- Unique in their ability to uncouple CMR and CBF:
- Cause a decrease in CMR
- However, also abolish autoregulation and cause cerebral arteriolar vasodilatation, leading to increased CBF
- The action is dose-dependent:
- Below 1 MAC both effects are approximately equal and CBF is unchanged
- Above 1 MAC the reduction in CMR is already maximal and CBF increases due to cerebral arteriolar vasodilatation
- Sevoflurane has the lowest vasodilatory potential of the volatile agents
- Order of vasodilating potency is halothane > enflurane > desflurane > isoflurane > sevoflurane
- Both potently dilates cerebral arteries and increases CMR
- As a result CBF significantly increases
- No significant effect on either CMR or CBF
- CBF will rise in the setting of opioid induced CO2 retention
- No significant effect on either CMR or CBF
Monitoring CBF
See section: Cerebral Blood Flow (CBF) & Oxygenation Monitoring