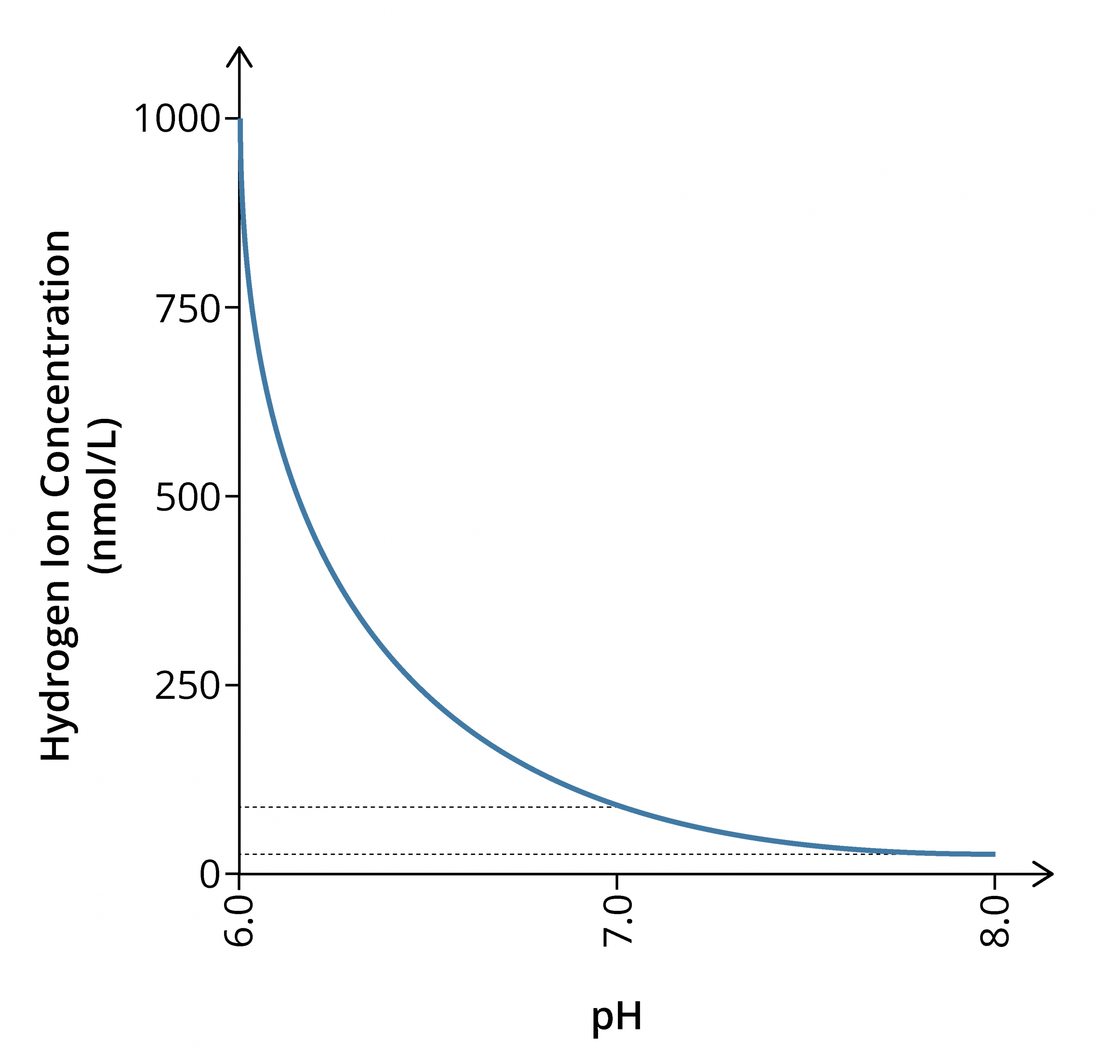
- The relationship between hydrogen ion concentration and pH is inverse
- As pH is a logarithmic function, there must be a 10-fold change in [H+] for each unit change in pH
- pH 8.0 – [H+] = 10 nmol/l
- pH 7.0 – [H+] = 100 nmol/l
- pH 6.0 – [H+] = 1000 nmol/l
- Normal hydrogen ion concentration [H+] in the blood is 40 nmol/l, giving a pH of 7.4