Time: 0 second
Question No. 2
Q: What are the functions of calcium in the body?
Answer No. 2
- Bone formation and metabolism:
- Contributes to strength and structure
- Role as a strong cation in acid-base balance
- Coagulation of blood (cofactor in coagulation pathway)
- Cellular functions:
- Excitation–contraction coupling in cardiac, skeletal and smooth muscle
- Cardiac action potentials and pacemaker activity
- Regulation of cell growth and apoptosis
- Cofactor for many enzymes (e.g. lipase) and proteins
- Membrane integrity & permeability
- Ciliary motility
- Cellular communication:
- Intracellular secondary messenger systems
- Secretory processes:
- Release of neurotransmitters
- Hormone release
- Catecholamine responsiveness
Question No. 3
Q: Which enzymes need calcium to function?
Answer No. 3
- The protein calmodulin activates many enzymes in the presence of calcium:
- Calcium-calmodulin complexes bind to enzymes acting as a co-factor
- Known as a 'calcium-binding regulatory protein'
- Enzymes activated by calmodulin include:
- Glycogen synthase
- Adenyl cyclase
- Pancreatic lipase
- Pyruvate carboxylase
- Phospholipase A2
Question No. 4
Q: Why it is needed for clotting?
Answer No. 4
- Ionized calcium is essential for many stages of both coagulation and platelet plug formation
- Multiple roles in coagulation pathway (Calcium is known as Factor IV):
- Required for the activation of factors VII, IX and X
- Acts as a co-factor in the conversion of prothrombin (II) to thrombin (IIa) - binds to the tenase enzyme where it acts as a catalyst speeding up the reaction
- Important in platelet activation and aggregation:
- Increase in intracellular calcium upon stimulation activates a number of calcium-dependent enzymes and regulated release of active substances
- Standard anticoagulant solutions contain citrate which chelates calcium ions and efficiently prevents clotting
Question No. 8
Q: How are calcium levels affected by changes in albumin concentration?
Answer No. 8
- Total calcium levels are generally dependent on the level of plasma albumin
- With reduced serum albumin proportionally less calcium is bound
- This results in a lower measured total plasma calcium
Question No. 9
Q: How can the affect of albumin levels be accounted for?
Answer No. 9
- Total calcium levels are generally dependent on the level of plasma albumin
- With reduced serum albumin proportionally less calcium is bound
- This results in a lower measured total plasma calcium
- A corrective formula (Payne's formula) is commonly applied to account for albumin concentrations when considering the measured total calcium levels
- A correction factor of 0.02 mmol/L is added to the measured calcium level for every 1 g/L of albumin below a value of 40 g/L)
- Results in the formula:
Corrected Ca2+ = measured Ca2+ + 0.02 x (40 – serum albumin)
- For example, if the measured total plasma calcium is 1.876 mmol/L and plasma albumin is 26 g/L:
| Corrected Ca2+ | = 1.83 + 0.02 x (40 – 26) = 1.83 + 0.28 = 2.11 mmol/L |
Worked Example
Question No. 10
Q: What role does calcium play in cell excitability?
Answer No. 10
- Ca2+ ions competes for binding sites on albumin with H+ ions
- As pH changes the proportion of calcium bound to albumin and freely ionised in the plasma varies
- For every 0.1 unit reduction in plasma pH, the albumin bound calcium decreases by 0.07 mmol/L and ionised calcium increases by 0.07 mmol/L
- Hyperventilation can lead to alkalosis, and thus reduced plasma ionised calcium:
- As ionised calcium determines clinical effects tetany may be precipitated
Question No. 11
Q: How does the ionised calcium alter with changes in pH?
Answer No. 11
- Ca2+ ions compete for binding sites on albumin with H+ ions
- As pH changes the proportion of calcium bound to albumin and freely ionised in the plasma varies
- For every 0.1 unit reduction in plasma pH, the albumin bound calcium decreases by 0.07 mmol/L and ionised calcium increases by 0.07 mmol/L
Question No. 12
Q: Why does tetany occur if you hyperventilate?
Answer No. 12
- Hyperventilation can lead to alkalosis, and thus reduced plasma ionised calcium:
-
- As ionised calcium determines clinical effects tetany may be precipitated
Question No. 13
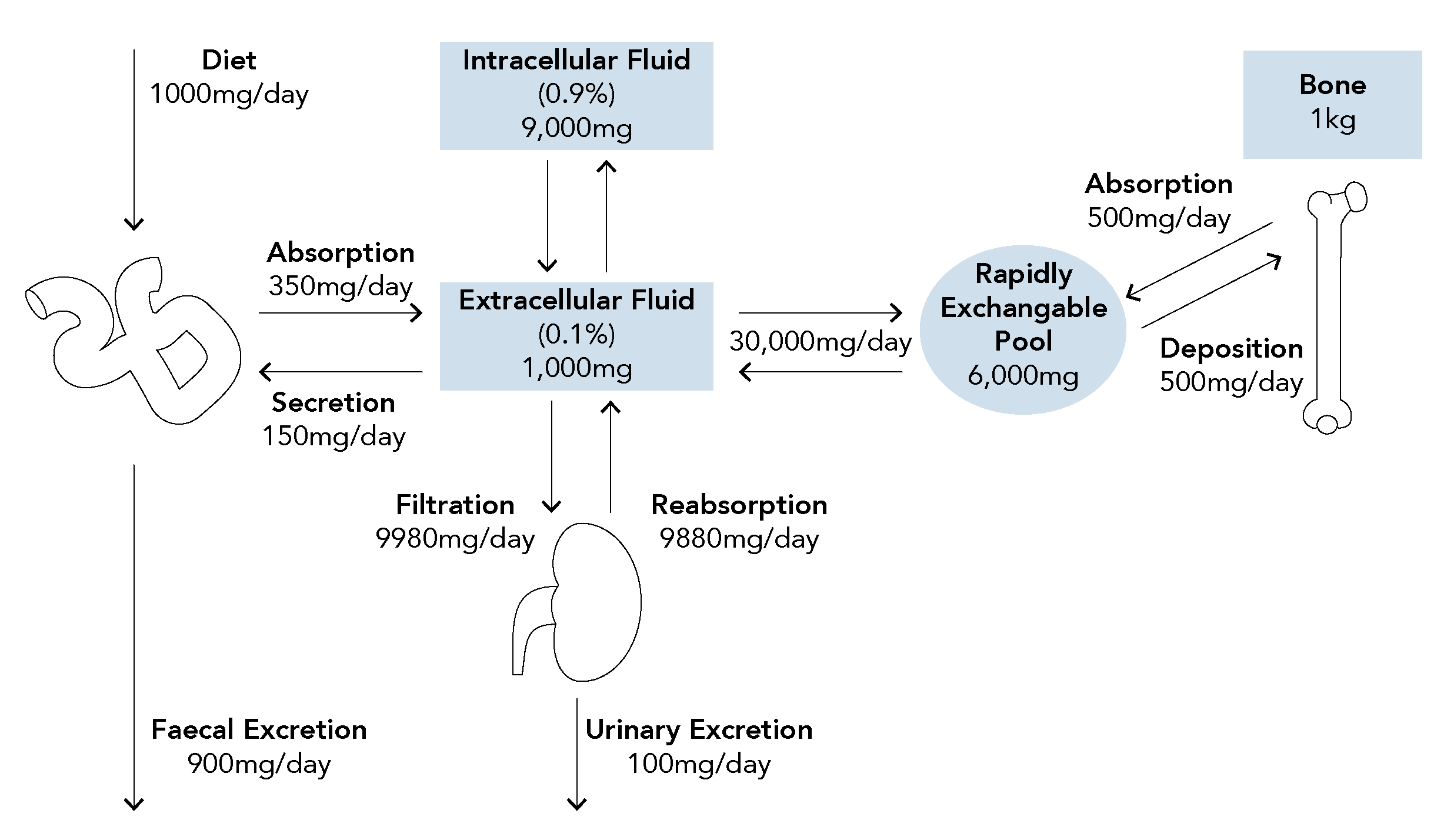
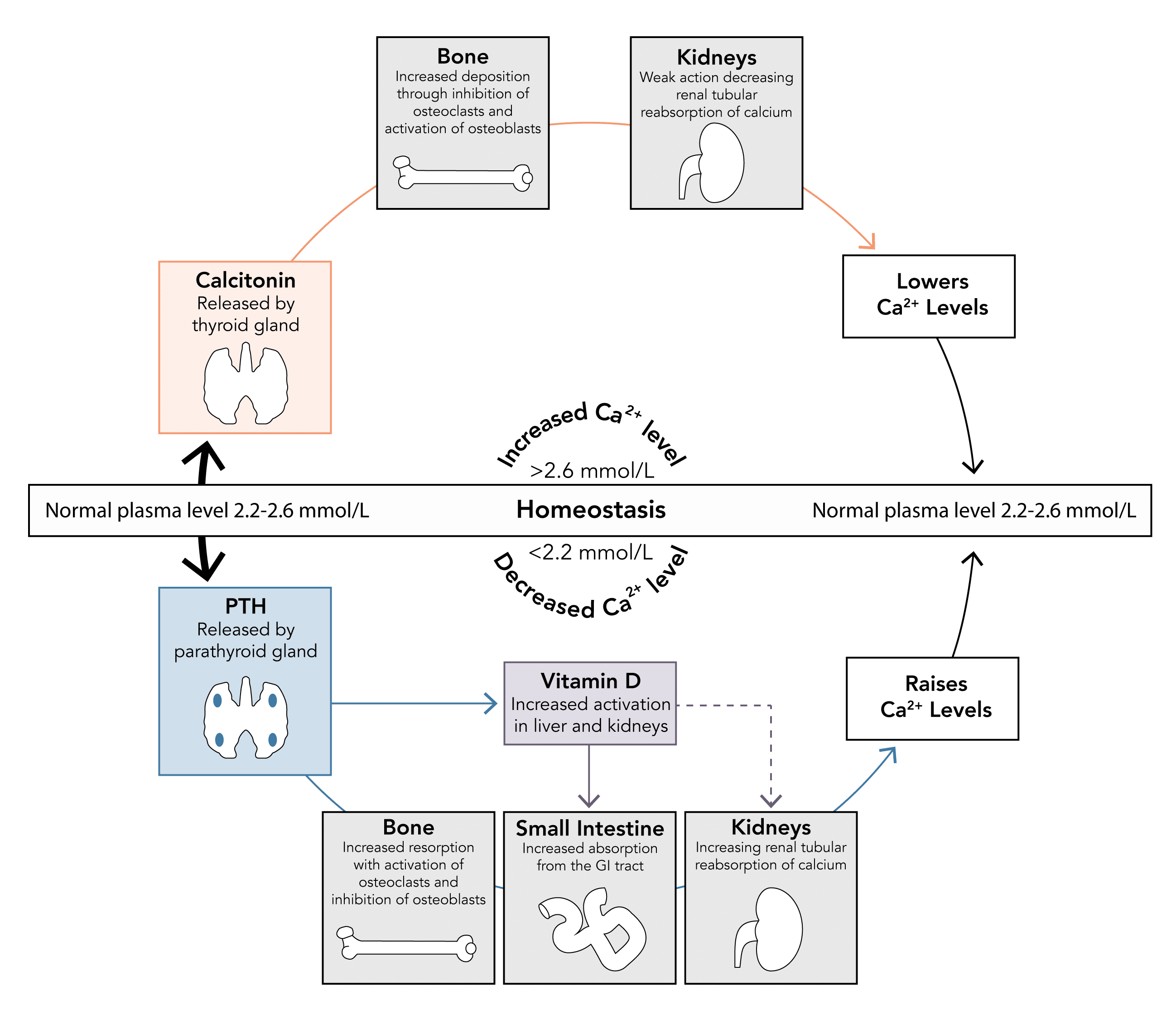
Q: How is calcium homeostasis achieved? Which organs and which hormones are important and what are their effect?
Question No. 14
Q: What is parathyroid hormone? Where is it produced, what are its effects?
Answer No. 14
Hormone
Hormone
Hormone
Parathyroid Hormone
- Protein hormone produced by chief cells of the parathyroid gland
- Secretion stimulated by a decrease in serum calcium levels
- Normal levels are 1.3 – 6.8 pmol/L
- Acts to increase serum calcium and decrease serum phosphate levels through a number of mechanisms:
- Kidney: Increases calcium reabsorption
- Bone: Mobilises calcium from bone stores by stimulating osteoclast activity and inhibiting osteoblast activity
- GI Tract: Increases GI absorption of calcium and phosphate indirectly by production active vitamin D metabolites
Question No. 16
Q: What are the effects of vitamin D?
Answer No. 16
Hormone
Hormone
Hormone
Vitamin D3
(cholecalciferol)
(cholecalciferol)
- Originates from:
- Dietary intake
- Synthesis in the skin through effects of UV light on 7-dehydrocholesterol
- Modified by liver and kidney to form active 1-25-hydroxycholecalciferol - under the influence of parathyroid hormone
- Levels also increase in response to low phosphate levels
- Acts to increase serum calcium levels
- GI Tract: Increases GI absorption by upregulating number and activity of transport proteins
- Kidneys: Weak effect to increase calcium and phosphate reabsorption
- Plays a minimal role in calcium homeostasis, secreted only when levels rise above normal limits
Question No. 17
Q: What is calcitonin? Where is it produced, what are its effects?
Answer No. 17
Hormone
Hormone
Hormone
Calcitonin
(minimal role)
(minimal role)
- Peptide hormone produced by parafollicular cells of the thyroid gland
- Secretion stimulated by an increase in serum calcium levels
- Acts to decrease serum calcium levels when elevated (physiological antagonist to PTH)
- Bone: Prevents resorption by inhibiting osteoclast activity and stimulating osteoblast activity
- Kidneys: Weak action to decrease calcium reabsorption