Time: 0 second
SOE 619: Fever & Lymphadenopathy
Introduction
An 18-year-old man is admitted to intensive care for vasopressor support. He has recently been assessed in the rheumatology clinic for worsening joint pains over the past 6 months. He presented with a 3-day history of fever, dyspnoea and lymphadenopathy. He is treated initially for sepsis with broad-spectrum antibiotics…
Question No. 4
Q: What do you consider as your differential diagnoses?
Answer No. 4
Infectious Disease
- Bacterial Infection (failure of antibiotic therapy due to organism, patient or drug factors)
- Viral infection (EBV, CMV, Parvovirus, HIV)
- Fungal infection
Inflammatory Disease
- Systemic juvenile idiopathic arthritis
- Kawasaki disease
- Systemic Lupus erythematosis
- Hemophagocytic lymphohistiocytosis (HLH)
Neoplastic Disease
- Lymphoma
- Acute lymphoblastic leukaemia (ALL)
Question No. 6
Q: What is hemophagocytic lymphohistiocytosis (HLH)?
Answer No. 6
- Hemophagocytic lymphohistiocytosis (HLH) is an aggressive and life-threatening hyperinflammatory syndrome, which requires prompt and aggressive treatment
- It is termed macrophage activation syndrome (MAS) when associated with rheumatic disease and secondary HLH (sHLH) when associated with other triggers, including malignancy and infection
- HLH represents a group of conditions with different pathogenetic roots but a shared common final pathway
- It most frequently affects infants up to 18 months of age but can be seen in children and adults of all ages
Question No. 7
Q: Why is HLH relevant to intensive care?
Answer No. 7
- Usually requires ICU management given the severity of clinical features
- Often first diagnosed in patients with known sepsis or multi-organ failure:
- Has non-specific symptoms and laboratory findings
- Requires ICU clinicians to have a high index of suspicion
- Has specific treatments available but a poor prognosis without
Question No. 8
Q: How common is HLH?
Answer No. 8
- Estimated at <1 per 100,000 children under 18
- Likely to be significantly under-recognised
- Incidence may be as high as 1 in 2000 adult admissions at tertiary medical centres
Question No. 9
Q: What is the prognosis of HLH?
Answer No. 9
- Primary HLH is almost universally fatal without treatment
- Mortality improved with treatment
- Approximately 50% with HLH-94 based treatment
- Secondary HLH and HLH in adults without treatment has high mortality:
- Overall mortality of 50% to 75%
- Stem cell transplant has resulted in significant improvements in long term survival and cure
Question No. 10
Q: What are the causes of HLH?
Answer No. 10
Primary
Primary
- Genetic defects – usually autosomal recessive:
- Function of cytotoxic T cells or NK cells
- Inflammasome regulation
Secondary (Acquired)
Secondary (Acquired)
Viral Infections
- EBV
- HSV
- HIV
- CMV
29%
Other Infections
- Bacterial
- Protozoal (Malaria, Leishmania)
- Fungal (Candida, Aspergillus)
- Mycobacteria
- Mycoplasma
20%
Malignancies:
- Natural killer (NK) cell lymphomas
- B-cell lymphomas
- Hodgkin lymphoma
- Leukaemia
- Other hematologic neoplasms
- Solid tumours
27%
Rheumatological Conditions
- Juvenile idiopathic arthritis
- SLE
- Adult-onset Still's Disease
- Rheumatoid Arthritis
7%
Immune Deficiency States
- Stem or bone marrow transplant
- Solid-organ transplant
- Severe combined / common variable immunodeficiency
6%
Other
- Haemodialysis
- Pregnancy
- Vaccination
Rare
Question No. 11
Q: What are the underlying pathophysiological mechanisms in HLH?
Answer No. 11
In the standard-setting:
- Cytotoxic T Lymphocytes (CTLs) and Natural Killer (NK) cells eliminate infected or tumour cells via apoptosis
- When the cells are cleared, the CTLs will inhibit further antigen presentation by removing antigen‐presenting Dendritic Cells (DCs)
- T-Regulatory Cells (Tregs) compete with and limit the proliferation of CTLs. They may also directly eliminate activated CTLs
- NK cells likewise control the size of the activated CTL pool via induction of apoptosis
- This limits the amount of CTL‐derived IFN‐γ. This is required for macrophage activation and additional cytokine production, and so this becomes limited.
In the setting of HLH:
- Dysregulated immune system unable to restrict the stimulatory effect of various triggers (due to single or combined defects):
- CTLs and NK cells fail to eliminate tumour cells or infected cells, which continue to replicate, resulting in persistent antigenemia
- CTLs no longer remove the antigen-presenting DCs, leading to prolonged and heightened antigen presentation
- T Regulatory Cells are unable to regulate CTLs due to imbalanced cytokines. T regulatory cell numbers drop, and CTLs continue to proliferate.
- NK fail to control the size of the activated CTL pool due to loss of cytotoxic activity
- The activated CTLs produce massive amounts of IFN‐γ:
- Induces excessive macrophage activation
- Directly provokes haem phagocytosis.
- Activated macrophages release vast amounts of pro-inflammatory cytokines (a 'cytokine storm'):
- Interleukins: (IL)-1, IL-6, IL-10, IL-12, IL-16, IL-18
- Tumour necrosis factor (TNF)
- Results in ongoing cycles of inflammation and cytokine release:
- Exacerbated by failure to clear cells via apoptosis resulting in necrosis and further inflammation
Question No. 12
Q: What are the common clinical features of HLH?
Answer No. 12
Febrile illness (prolonged) associated with multiple organ involvement:
- Reticuloendothelial manifestations:
- Hepatosplenomegaly (95%)
- Lymphadenopathy (33%)
- CNS dysfunction (35%):
- Seizures
- Cranial nerve palsy
- Altered sensorium
- Respiratory dysfunction:
- Respiratory failure
- Alveolar / interstitial infiltrates
- Cutaneous manifestations (up to 65%)
- Maculopapular erythematous rashes
- Generalised erythroderma
- Oedema
- Panniculitis
Question No. 13
Q: What are the common laboratory features of HLH?
Answer No. 13
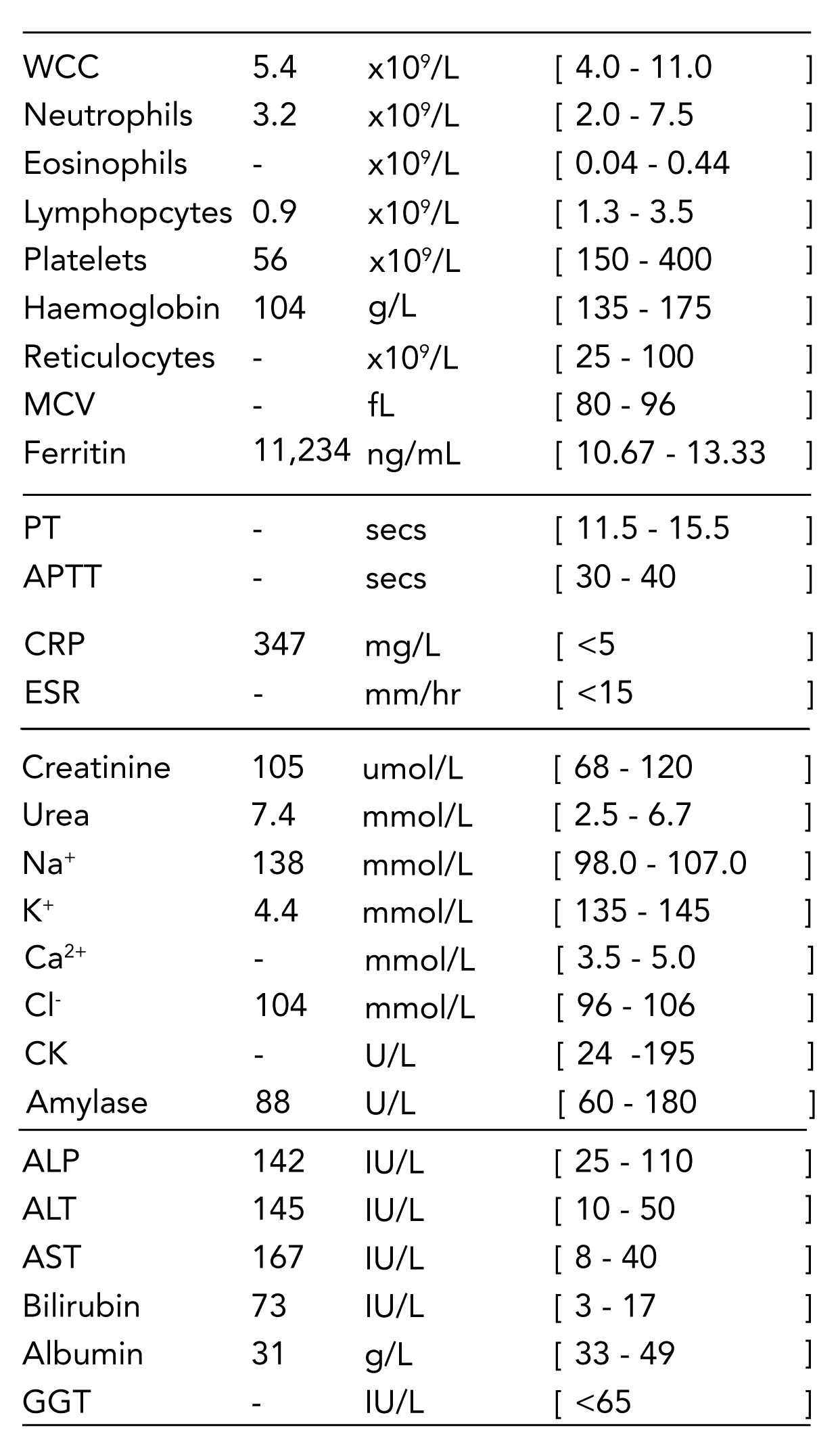
- High serum ferritin (>500, usually higher)
- Haematological abnormalities:
- Thrombocytopenia
- Anaemia
- Coagulopathy
- Hypofibrinogenemia
- Deranged LFTs:
- Hyperbilirubinaemia
- Transaminitis
- Hypertriglyceridemia
Question No. 14
Q: When should you suspect HLH in the ICU?
Answer No. 14
- Diagnosis can be challenging to make:
- Features are non-specific
- Physiological macrophage activation occurs in sepsis, malignancy and autoinflammatory disorders
- HLH is characterised by pathological macrophage activation
- Consider HLH in any critically ill patient with an inadequate response to treatment or unusual progression of symptoms:
- Persistent fever
- Unresponsive to vasopressors
- Inexplicable cytopenias
- Organ failure not responding to appropriate therapy and aggressive supportive care
Question No. 15
Q: How do you work-up the patient with suspected HLH?
Answer No. 15
To Determine Diagnosis
- Clinical history and findings
- Use of Clinical Scores:
- HLH Diagnostic Criteria (primary HLH)
- H-Score (secondary HLH)
- Laboratory evaluation:
- FBC
- Ferritin
- Fasting triglycerides
- Coagulation screen (fibrinogen, PT, aPTT)
- LFTs, LDH, albumin
- Immunological testing
- Bone marrow biopsy
Definitive testing:
If suspected, do not wait to treat!
If suspected, do not wait to treat!
- ADAMTS13 activity
- Anti-ADAMTS13 Ab
- Sequencing of ADAMTS13 gene
To Determine Aetiology
- Infection work-up:
- Relevant imaging
- Bacterial and viral studies
- Cancer work-up:
- PET-CT
- Tissue biopsy
- Tumour markers
Question No. 16
Q: What levels of ferritin are seen in HLH?
Answer No. 16
- The Texas children's study (Allen et al) suggested:
- >500 has 100% sensitivity
- >10,000 has 90% sensitivity and 96% specificity
Question No. 17
Q: How can HLH be diagnosed?
Answer No. 17
- Should be diagnosed based upon clinical judgement and history in conjunction with a diagnostic score/criterion
- Scoring systems available include:
HLH Diagnostic Criteria
- Produced in 2004 by the histiocyte society
- Developed for diagnosis of primary HLH in paediatric population
- Frequently applied to adult population though poorly validated
H-score
- Weighted criteria developed in 2014
- Only validated for secondary forms of HLH in adults
Question No. 18
Q: What is the diagnostic criteria for HLH?
Answer No. 18
The 2004 revision of the diagnostic criteria for HLH requires:
- Molecular testing consistent with HLH
or - 5 of 8 clinical or laboratory criteria
Clinical / Laboratory Criteria
- Fever
- Splenomegaly
- Cytopenias of at least 2 cell lines
- Hypertriglyceridemia
- Hypofibrinogenemia
- Elevated ferritin
- Elevated soluble IL-2 receptor (sCD25)
- Decreased or absent NK-cell activity
- Demonstration of hemophagocytosis in bone marrow, spleen, or lymph nodes
Supportive But Not Definitive Criteria
- Elevated transaminases
- Elevated bilirubin
- Elevated LDH
- CSF pleocytosis and/or elevated protein
Question No. 19
Q: What is the H-score?
Answer No. 19
- A set of weighted criteria, producing a score out of 337
- Score ≥169 commonly used as a cut-off as a likelihood for a diagnosis of HLH
- Produces a % risk of HLH based upon weighted score
No. of Points
Parameter
Parameter
No. of Points
(Criteria for scoring)
(Criteria for scoring)
Known Underlying Immunosuppression
(HIV or Immunosuppressive therapy)
0 (no)
18 (yes)
18 (yes)
Fever (C)
0 (<38.4)
33 (38.4-39.4)
49 (>39.4)
33 (38.4-39.4)
49 (>39.4)
Organomegaly
0 (no)
23 (hepatomegaly or splenomegaly)
38 (hepatomegaly and splenomegaly)
23 (hepatomegaly or splenomegaly)
38 (hepatomegaly and splenomegaly)
Number of Cytopenia
(Hb <9.2 g/L / WBC ≤5 x 109/L / plat ≤110 x 109/L)
0 (one lineage)
24 (two lineages)
34 (three lineages)
24 (two lineages)
34 (three lineages)
Triglycerides (mmol/L)
0 (<1.5)
44 (1.5-4)
64 (>4)
44 (1.5-4)
64 (>4)
Ferritin (µg/L)
0 (<2000)
35 (2000-6000)
50 (>6000)
35 (2000-6000)
50 (>6000)
Question No. 20
Q: What is the ICU management of HLH?
Answer No. 20
Key Principles
- Provision of supportive care for organ failure
- MDT approach towards management
- Treatment of underlying cause
- Specific management based on HLH-94 protocol
Initial Resuscitation & Supportive Care
- Aggressive approach to organ support - may require:
- Invasive ventilation
- Vasopressor support
- Renal replacement therapy
Specific Management
- Treat the underlying cause:
- Rheumatological - corticosteroids
- Infectious - antimicrobials
- For primary / refractory secondary disease HLH therapy based on the HLH-94 protocol:
- Etoposide
- Dexamethasone
- Intrathecal methotrexate
- If failure to respond additional treatments may be required haemopoietic stem cell transplant
Referral & Deposition
- Senior MDT approach - input from:
- Haematology
- Rheumatology
- Cardiology
- Microbiology / Infectious Diseases
Question No. 21
Q: Which drugs have been used in the management of HLH?
Answer No. 21
The classical HLH-94 protocol includes:
- Corticosteroids - usually dexamethasone in primary forms and methylprednisolone in secondary forms
- Etoposide - a chemotherapy agent with high specificity against T-cells
- Cyclosporin A
- Methotrexate
Other drugs that are commonly used include
- Anakinra (an interleukin-1 inhibitor)
- Immunoglobulin
- Alemtuzumab
- Tocilizumab