Time: 0 second
Question No. 2
Q: What is thrombotic thrombocytopenic purpura (TTP)?
Answer No. 2
- Thrombotic thrombocytopenic purpura (TTP) is a rare thrombotic microangiopathy resulting in a consumptive thrombocytopenia, haemolytic anaemia and ischaemic end-organ damage
- It occurs due to a deficiency in the activity of a specific von Willebrand factor cleaving protein (vWF-CF) known as ADAMTS13
- It is an intensive care and haematological emergency
Question No. 3
Q: How common is TTP?
Answer No. 3
- TTP is a rare condition:
- Prevalence is only 10-15 per 1,000,0000
- Low incidence hinders the development of strong evidence base
- Has a female preponderance with a ratio of 2:1
- Peak incidence is in adulthood before the age of 50
Question No. 4
Q: What is the prognosis of patients with TTP?
Answer No. 4
- Untreated the mortality is 90%
- The current mortality in the UK (from the TTP register) is 10-20%)
- Has significantly decreased since the introduction of early PEX
- Most deaths from cardiac and neurological complications
- Relapse rate is 30-50%
Question No. 5
Q: Which factors are associated with worse outcomes in TTP?
Answer No. 5
-
Age >60
-
Elevated troponin
-
CNS involvement
-
Delayed diagnosis
-
Use of platelet transfusion
-
Elevated LDH
-
Refractory disease unresponsive to PLEX
-
Episodes of relapse
-
African or Caribbean ethnicity
Question No. 6
Q: What is the pathophysiology of TTP?
Answer No. 6
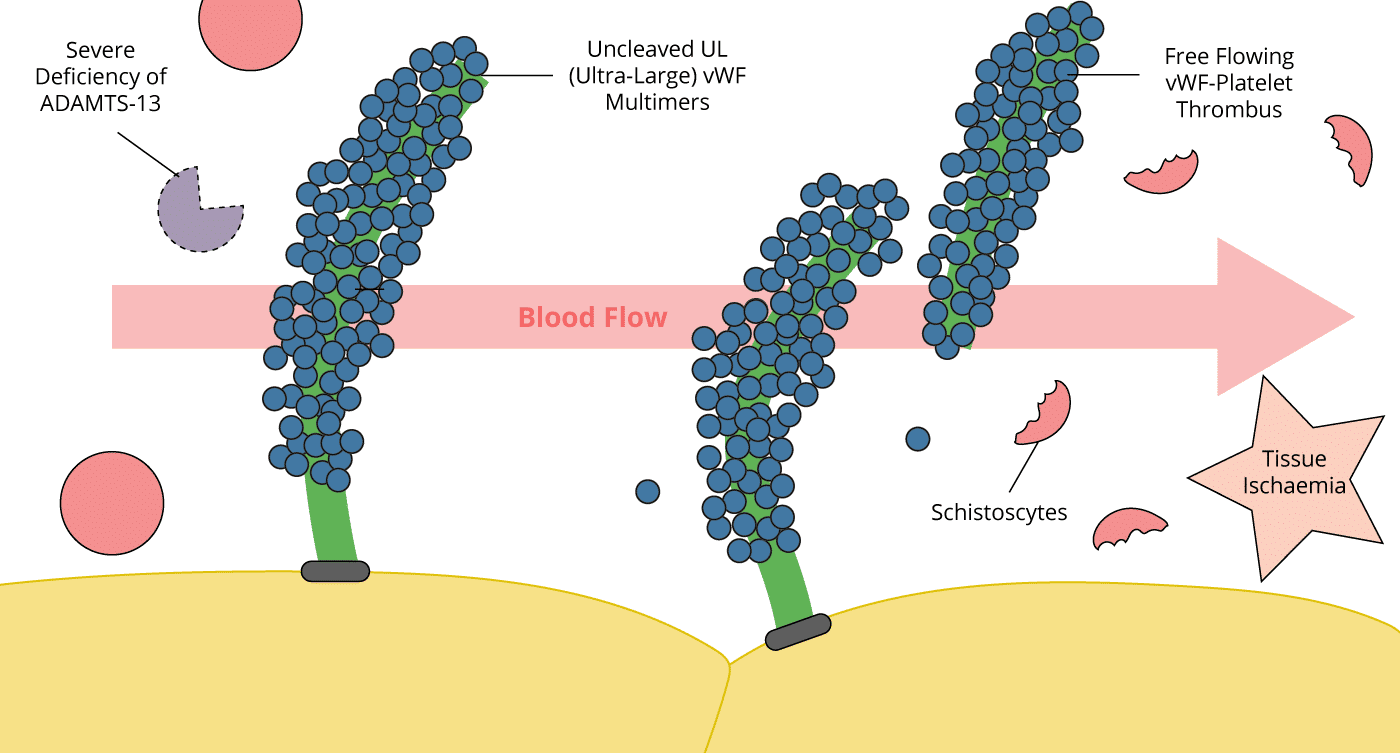
- vWF is a large glycoprotein present in the plasma whose functions include binding factor VIII, and activating and binding platelets in response to endothelial injury.
- It is produced in the endothelium as ultra-large multimers that are inactivated when cleaved by Von-Willebrand factor-cleaving protease (vWF-CP), also known as ADAMTS13 (A Disintegrin And Metalloprotease with a ThromboSpondin type 1 motif, member 13
- The pathological hallmark in TTP is a deficiency of von Willebrand factor-cleaving protease (vWF-CP) or ADAMTS13. This may be genetic (absence of enzyme) or acquired (presence of autoantibody to vWF-CP).
- In TTP, these multimers are not cleaved resulting in ultra-large multimers
- The VWF multimers bind to platelets and result in uncontrolled platelet activation. Fibrin is deposited and thrombus propagated
- The end result is ischaemia distally, and red cells are shredded as they pass the fibrin/platelet mesh (microangiopathic haemolytic anaemia, MAHA).
Question No. 7
Q: What are the causes of TTP?
Answer No. 7
Primary
- Congenital (5%) - typically present in late infancy or childhood
- Acquired idiopathic (autoimmune)
Secondary
(To a known Trigger)
- Infection:
- HIV
- CMV
- HBV / HCV
- Pregnancy
- Bone marrow transplant
- Drugs:
- COCP and hormonal treatments
- Quinine
- Interferon and immunosuppressants
- Simvastatin
- Antiplatelets
- Malignancy
- Acute pancreatitis
Question No. 8
Q: How does TTP present?
Answer No. 8
- TTP is said to present with a classic pentad of features:
- However, not all features are required to be present to make a diagnosis
- Additional features are also commonly seen in TTP
- Patients report feeling unwell for several weeks before presenting:
- Usually, experience flu-like symptoms
- Relapses present much quicker
Classic 'Diagnostic' Pentad
- Microangiopathic haemolytic anaemia
- Thrombocytopaenia - often initial diagnostic feature
- Acute kidney injury (35%)
- Fever (25%)
- Neurological dysfunction (80%) - Altered mental state, headaches, confusion, seizures, intracranial haemorrhage, focal deficits
Additional Features
- Cardiac dysfunction (40%) - elevated troponin, large territory ECG changes not common as microvascular involvement
- GI dysfunction (35%)
- Flu-like symptoms - often experienced for several weeks before presentation
Question No. 9
Q: What are the laboratory features of TTP?
Answer No. 9
- Features of MAHA:
- Anaemia on FBC
- Blood film: low platelets, schistocytes, polychromasia
- Increased reticulocytes, bilirubin and LDH
- Negative DAT Coomb's test
- Marked thrombocytopenia
- Decreased ADAMTS13 activity
- Anti-ADAMTS13 IgG may be identified
Question No. 10
Q: What are the features of severe disease?
Answer No. 10
Severe disease is complicated by organ failure:
Neurological
- Seizures
- Paralysis
- Ischaemic Changes
- Cerebral Bleeding
- Coma
Renal
- Renal Failure
Cardiovascular
- Acute myocardial infarction
- Arrhythmias
- Heart failure
- Cardiogenic shock
Other organs
- Pancreatitis
- GI Bleeding
- Mesenteric Ischaemia
Question No. 11
Q: What are the differential diagnoses for TTP?
Answer No. 11
Disease
Pathophysiology
Differentiating Features
Other Thrombotic Microangiopathies
Other Thrombotic Microangiopathies
Other Thrombotic Microangiopathies
DIC
Inflammatory mediated consumptive coagulopathy
- History of infection, malignancy or predisposing factors
- Low fibrinogen
- Schistocytes may be absent
HELLP
Inflammatory mediated consumptive coagulopathy
- Hypertension and proteinuria always present
- Seizures prominent
- LFTs usually elevated
- Schistocytes may be absent
Other
Other
Other
ITP
Immune mediated platelet destruction
- Clinically well
HIT
Immune mediated platelet destruction
- History of heparin exposure
- No evidence of haemolytic anaemia
- Large vessel thrombosis may be present
- Anti-PF4 antibodies may be identified
Heart Valve Haemolysis
Mechanical fragmentation of red blood cells with consumption of platelets
- History of mechanical heart valve
- Heart valve defect on echocardiography
Evan's Syndrome
Immune thrombocytopenia with Coombs positive autoimmune haemolysis
- Schistocytes absent
- Positive Coombs test
Endocarditis
Likely combination of mechanical fragmentation and consumptive coagulopathy
- Vegetations on echocardiography
- Positive blood cultures
Catastrophic anti-phospholipid syndrome
Arterial and venous thrombi with secondary endothelial damage
- Prolonged aPTT
- Positive cardiolipin antibody
Question No. 12
Q: How do you differentiate TTP and haemolytic uraemic syndrome (HUS)?
Answer No. 12
- Can be difficult as share similar clinical and pathophysiological features
- Differentiating clinical features include:
- ↑ presence of focal neurological symptoms in TTP
- ↑ presence of renal failure in HUS
- Typical HUS:
- Associated with exposure to shiga toxin - may be preceded by history of bloody diarrhoea
- Commonly presents in children <5
- Is associated with a higher platelet count than TTP (usually >35 x 109/L
Question No. 13
Q: How is TTP Diagnosed?
Answer No. 13
- The hallmark of TTP is absence of ADAMTS13 activity
- A level <5–10% is now required to confirm the diagnosis
Question No. 14
Q: How can ADAMTS13 activity deficiency be detected?
Answer No. 14
- ADAMTS13 activity is tested using specialist functional assays which are conducted at reference centres:
- Principle involves degradation of vWF substrate (either full length or small peptides)
- The value is expressed as a % compared against activity from normal pooled plasma which is considered as 100%
Question No. 15
Q: What is the management of TTP?
Answer No. 15
Key Principles
- Early resuscitation and supportive care
- Urgent and rapid treatment or transfer to specialist centre (Treat as urgent as aneurysm)
- Avoidance of platelet transfusion
- Early plasma exchange with FFP
- Immunosuppressive therapy
- Therapy to reduce thrombosis
Initial Resuscitation & Supportive Care
- ABC approach treating abnormalities as found:
- 100% oxygen whilst assessing
- Obtain IV access and perform diagnostic work-up
- Consider early central line and vascath for plasma exchange
- May need intubation if significant neurological sequalae
- Management of seizures using benzodiazepines
- Monitor urine output and consider RRT if evidence of
- IV PPI for patients whilst on high dose steroids
- For management of haemolysis:
- Transfuse to target of 70g/dL
- Commence oral folic acid 5mg OD
Specific Management
- Platelet transfusions contraindicated unless Major haemorrhage:
- Worsens thrombosis
- Usually prothrombotic - lines can be performed without
- Plasma exchange - Mainstay of treatment:
- Removes the autoantibodies from the patient's circulation, and replaces their plasma with plasma containing normal levels of vWF-CP
- Ideally instigated within 3-4 hours of diagnosis
- Using Octaplas (solvent-detergent prepared FFP deficient in ultra-large multimeric vWF)
- Daily PEx should continue for at least 2 days after platelet recovery (i.e. pits >150 x 109/L)
- FFP may be given has holding measure whilst awaiting transfer:
- Not replacement for PLEX
- Dose 15ml/Kg
- Immunomodulatory therapy
- IV Methylprednisolone 1g for 3 days immediately after PLEX
- Additional therapy (severe or refractory disease)
- Rituximab (monoclonal antibody against CD20, found on the surface of B cells)
- MMF
- Azathioprine
- Therapy to reduce thrombosis
- Aspirin 75mg Once plt >50 x 109/L
- Prophylactic LMWH once plt >50 x 109/L
Referral & Deposition
- Needs urgent liaison with haematology if suspected
- Arrange rapid transfer to specialist centre:
- Always blue light (Agreement with regional ambulance services)
- New cases - manage on HDU if not on ICU
Question No. 16
Q: Which patients with TTP should be admitted to critical care?
Answer No. 16
Question No. 17
Q: Which centres should patients with TTP be managed at?
Answer No. 17
- Given the very low incidence of TTP suggested that TTP managed at a regional referral centre:
- Have multidisciplinary teams with experience in ICU and long- term aspects of management
- National agreement now in place with Regional Ambulance Services that TTP forms one of nine conditions designated as a ‘critical transfer’ requiring immediate life‐saving transfer:
- Due to risk of deterioration anaesthetic escort is advised to avoid arrival of unstable patients.
Question No. 18
Q: How does PLEX work in TTP?
Answer No. 18
- Removed autoantibodies to ADAMTS13
- Replaces ADAMTS13 in plasma
Question No. 19
Q: How should plasma exchange (PLEX) be performed in TTP?
Answer No. 19
- Should be initiated as soon as possible (preferably within 4-8 hours of diagnosis):
- Early initiation associated with better outcomes
- May require emergency transfer to specialist centre
- Should not wait for result of ADAMTSq3 activity assay
- Ideally daily spun apheresis should be performed:
- Initially, 1.5x plasma volume should be exchanged for 3 days
- Then 1x plasma volume can be exchange
- Once platelet count >150 x 109/L for 2 days consecutively can be discontinued
- Duration of PLEX required to achieve remission is often highly variable
- Detergent treated FFP should be used to reduce the risk of transfusion-transmitted infection